INTRODUCTION
Advancements in aquaculture have increased in the past years as an answer to the increasing demand for food for the ballooning human population (1, 2). In attempts to maximize yield and production, such as by increasing stock densities in smaller holding capacities, as in intensive aquaculture systems, the frequency of disease outbreaks also increased, resulting in economic detriment and mass mortality (3, 4). Antibiotics have been utilized to mitigate such problems, but the overuse and misuse have ultimately caused another problem: the proliferation of antimicrobial-resistant bacteria (2). Such bacteria have developed resistance mechanisms, such as drug modification, efflux pumps, and biofilm formation, that allow them to override the activity of antimicrobials for disease control (5, 6). Apart from probing for novel sources of antimicrobial compounds, attempts have been made to search for methodical substitutes, ranging from bacteriophage therapy, nanoparticles, adjuvants, inhibitor molecules, and probiotics, with probiotics gaining popularity in recent scientific pursuits (7, 8, 9, 10).
In fish, as part of its innate immune system, the mucosa is the frontline defense against disease, whether due to biological or environmental stresses (7, 11). Such slimy secretion acts as a natural and biochemical barrier that confers protection to the host in two ways- the regular shedding prevents the stable settlement of the pathogens in the mucus due to its antiadhesion properties, and second, suspended bioactive compounds, such as immunoglobulins, lysozymes, proteases, antimicrobial peptides, and lectins act in concert and participate in immune signaling cascades against invading pathogens (3, 12, 13). The mucus is produced in epithelial tissues of the skin, gut, buccal cavity, nasopharynx, and gills (14). This information elicited research work on the bioactive properties of the mucosa and the immunocompetent molecules present in it. Apart from the defensive properties in the host, it also aids in nutrient and gas exchange, sequestering hormones, protecting gametes, regulating osmotic and ionic concentrations, and, to some extent, in parental care (13, 15, 16, 17, 18).
Further, as a nutrient-rich environment, the mucus is a unique transitional zone between the host fish and the external environment. It is able to cater to a unique community of microbial species composed of pathogenic, commensal, and symbiotic groups (19, 20); the imbalance in the community structure of such groups can make the host more vulnerable to disease (21, 22). While the mucus is notorious against pathogenic species, it is able to tolerate some commensal and symbiotic groups that reinforce the protection provided by the mucosal lining and contribute to the health of the fish (7, 17, 23, 24). With their contribution through immunomodulation and rebiosis, such aspects can be harnessed for the isolation of probionts with potential use in aquaculture.
Although there have been studies on the mucosa, there still exists a dearth of understanding with regard to the mucosa as a subject of research in fish health. It is also an untapped reservoir of bioactive compounds and potential probionts on account of its physiological immune function. With this, the paper revisits the bioactive properties of the fish mucosa and uncovers its potential to be a source of bioactive compounds and probiotics. Studies on probiotics from mucosal layers are relatively scarce although the protection on account of their presence and contribution to the immune organ are imperative. With the increasing need for novel solutions and alternatives for disease management in aquaculture, the synthesis that this review provides can advance more research on the exploration of the mucosa in fish health. Specifically, the review covered the (a) bioactivity of the mucosal secretion in in vitro and in vivo works, (b) the bioactive compounds derived from the mucus, and (c) probiotic candidates that have been isolated.
BIOACTIVITY OF THE MUCOSAL SECRETION
Mucus in Host Defense
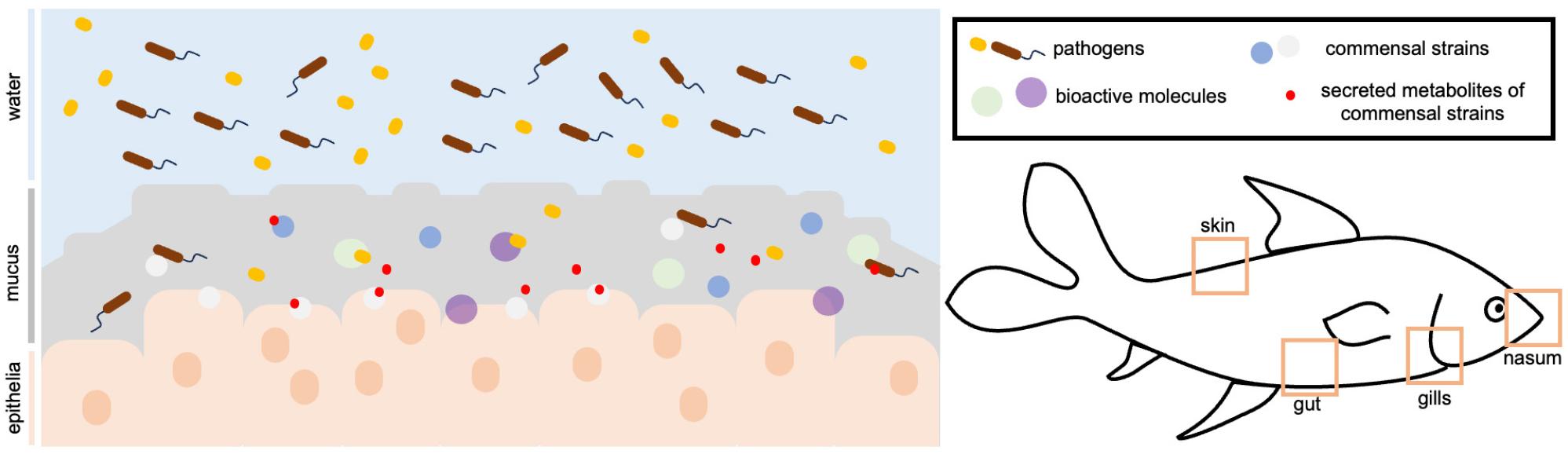
In fish physiology, the mucus is the first line of biological barrier against disturbances that may result in disease. With a less developed adaptive immune system, fish rely on their innate immunity, which includes the mucosal layers (16, 25). As a physical barrier, its multimeric structure of proteins traps pathogens and removes them from the mucosa during shedding (10). As a chemical barrier, it is able to sequester biomolecules that can act against these pathogens that invade the mucosa (3). In the host, various factors in the environment prompt mucus production, such as the presence of pollutants, pathogenic infections, food deprivation, and immune system compromise. Mucus expression is a response to such biological and environmental stresses as an attempt to counteract dysbiosis (25, 26, 27). Such response may be through the upregulation of specific metabolites, as observed in the study of Fernandez-Alacid et al. (2018) (26). By stimulating different environmental challenges posed throughout the life cycle of fish, namely, food deprivation, air exposure, and pathogenic infection, there were consistent trends in metabolite production in the mucus, which can be used as non-invasive biomarkers to monitor physiological responses brought about by changes in the environment (13, 25). The involvement of the mucus in fish health is axiomatic. As an overview, the mucus is composed of biotic components, such as microbes, whether pathogenic, commensal, or symbiotic, as well as abiotic components, such as bioactive and structural molecules (Fig. 1). The interactions among these components shape mucosal health. In the mucus, pathogenic groups are regulated by the presence of bioactive molecules along with the contributions from the commensal strains, mitigating disease and imbalance. The exploration of these components will be tackled in the succeeding sections of the review.
Bioactive Properties of the Mucus Extracts
With its intrinsic involvement as a defensive organ in the fish host, the earliest investigations on the bioactive properties of fish mucus extracts revolved around their antibacterial properties against various fish and human pathogens tested in vitro. Subramanian et al. (2008) initiated studies on the antimicrobial activity of acidic mucus extracts (28). Among the fish species tested, the mucus extracts of the brook trout (Salvelinus fontinalis), haddock (Melanogrammus aeglefinus), and hagfish (Myxine glutinosa) showed broad activity. Based on the minimum bactericidal concentrations (MBC), differential activity was observed for each species: the brook trout had the lowest MBC against a human pathogen, Salmonella enterica, at 10.0 ug/mL of extract. The haddock acidic mucus extract had an MBC of 14.0 ug/mL against human pathogens, S. enterica and E. coli, and against fish pathogen Yersinia ruckeri; and lastly, the mucus of the hagfish was able to inhibit E. coli and Y. ruckeri at a minimal concentration of 6.1 ug/mL.
Bragadeeswaran and Thangaraj (2011) investigated the hemolytic and antibacterial activity of the crude, aqueous, and methanolic extracts of the skin mucus of the common eel, Anguilla anguilla (11). A hemolytic assay using chicken and goat blood and diffusion assay were implored to investigate such properties. The mucus was found to have hemolytic and inhibitory activity against various shrimp pond pathogens. Notably, the crude extract had the highest inhibition against Salmonella paratyphi. In the study of Del Rosario et al. (2012), the chame fish (Dormitator latifrons) mucus showed significant inhibition against various Vibrio species (29).
Dhanaraj et al. (2009), meanwhile, explored the antibacterial properties of the skin and intestinal mucus of snakehead fish (Channa sp.) against various pathogens (30). In the study, the skin mucus was most effective against Vibrio fischeri, while the gut mucus inhibited Pseudomonas aeruginosa the most. Kuppulakshmi et al. (2008), in addition, found out that the snakehead mucus was effective in inhibiting Vibrio cholerae (31). Wei et al. (2010) investigated similar properties with varying methods of extraction of the mucus (32). Crude and aqueous extracts were potent against Aeromonas hydrophila, while acidic extracts were able to inhibit human pathogens, Klebsiella pneumoniae, P. aeruginosa, and Bacillus subtilis. Additionally, Hussain and Sachan (2024) studied the antimicrobial efficiency of the snakehead fish mucus (Channa punctatus), and the results of their study indicate that the mucus outperformed the positive antibiotic controls in inhibiting the growth of Staphylococcus aureus, E. coli, and fungus Hypericum gramineum(33). Lirio et al. (2019) also agree with the antimicrobial activity of snakehead fish (Channa striata) mucus in their evaluation against medically important pathogens (34). In their study, however, comparing the snakehead mucus with the catfish (Clarias batrachus) and tilapia (Oreochromis niloticus) mucus, the catfish mucus presented the highest inhibition against the tested pathogens, P. aeruginosa, K. pneumoniae, Enterococcus faecalis, Micrococcus luteus, A. hydrophila, S. aureus, E. coli, Serratia marcescens, with the lowest minimum inhibitory concentration (MIC) against S. aureus and M. luteus.
Apart from inhibiting the growth of bacterial pathogens, the fish mucus also has antifungal activity. In the study of Pethkar and Lokhande (2017), the skin mucus of the Indian carp (Catla catla), mrigal (Cirrhinus mrigala), and common eel (Anguilla anguilla) were able to inhibit the fungal growth of phytopathogenic species, Aspergillus awamori and Colletotrichum falcatum (35). The antifungal and antibacterial properties of the skin mucus of the swamp eel (Monopterus albus) were also evaluated by Ikram and Ridzwan (2013). They tested its skin mucus against fungal species, Candida albicans,Candida krusei, Cryptococcus neoformans, and Fusarium spp., with Fusarium spp. being the most sensitive to the mucus extract (36). Hilles et al. (2019) also reported that the skin mucus of the swamp eel decreased the growth of fungal species, Aspergillus niger and Microsporum gypseum(37).
The presented studies, thus far, substantiate the ability of the fish mucus to inhibit the growth of various microbial species that infect fish and humans, making the mucus a potent antimicrobial agent. However, the majority of the studies collected the skin mucus from apparently healthy fish. Mucosal production is usually a response to stress, and its bioactive components are upregulated under such conditions. To simulate the activity of the mucus in natural settings when the fish is subjected to environmental inconveniences, Ali et al. (2023) utilized different fish species, the Indian carp (Catla catla), mrigal (Cirrhinus mrigala), rohu (Labeo rohita), the grass carp (Ctenopharyngodon idella), and the silver carp (Hypophthalmichthys molitrix) (38). The fish were subjected to a treatment of A. hydrophila before mucus collection to simulate a natural infection under normal environmental conditions. Mucus samples collected post-treatment were subjected to acidic extraction and tested against various bacterial pathogens. Different extracts exhibited inhibition, even greater than their control antibiotic, fosfomycin, against different groups of bacterial species. While the modes of action of antibiotics are different for different pathogens, which in part yield lower inhibition to certain species as a component of innate immunity, the abovementioned studies establish the broad non-specific activity of the mucus against a wide roster of pathogenic species due to the presence of a wide repertoire of immunocompetent molecules that can counteract the vast number of pathogens that entangle in the mucus represented by the indicator strains. The effectiveness of the mucus extract of one fish species is different from one pathogen to another, as presented in the vast permutations of bacterial groups in which the fish mucus inhibited (28, 30, 31, 38).
Further, there was an observed differential in the antimicrobial activity of the mucus between indigenous and exotic species. For instance, in the study of Balasubramanian et al. (2012), indigenous fishes catla (Catla catla) and rohu (Labeo rohita) had greater antibacterial and antifungal activity observed than the silver carp (Hypophthalmichthys molitrix) and grass carp (Ctenopharyngodon idella), which are exotic fishes (39). All these are cultivable fish in India. Even the mucus obtained from the same fish species reared in different environments presented differences in antibacterial activity. In the study of Sajorne and Mabuhay-Omar (2020), the mucus obtained from the tilapia (Oreochromis spp.) raised in a fish pond had a higher antibacterial activity compared to the tilapia from a fish tank (40). This is speculated to be consequential of the more variable environmental conditions in the fish pond necessitating the fish to secrete mucus with more potent activity than the ones in the fish tank with more regulated environmental conditions.
Some studies have also considered using mucosal extracts as ingredients for formulations tested on animal models and have shown some promise. Hilles et al. (2022) studied the therapeutic potential of the skin mucus of the swamp eel evaluated with in vivo experimentation in mice (41). The skin mucus was formulated into a gel, which was administered to the mice intracutaneously injected with common bacterial (Streptococcus pyogenes, Staphylococcus aureus) and fungal (Microsporum gypsum, Candida albicans) pathogens associated with topical infections. Remarkably, the formulation had comparative results with positive control of commercial antifungals and antibacterials.
Beyond the antimicrobial activity offered by the mucus against pathogens, some researchers have also attempted to explore other bioactive properties of the mucus. Patel et al. (2020) explored the antibacterial, antibiofilm, and antiadhesion properties of the mucus extract of the pool barb (Puntius sophore) (42). Apart from its synergistic antibacterial effect when combined with gentamicin against E. coli, P. aeruginosa, and Bacillus subtilis, the extract was able to compromise the structural integrity of the biofilm matrix, affecting the viability of the bacterial cells as determined by a series of assays through the reduction of the exopolysaccharide content. Biofilm formation is one of the mechanisms of the spread of antimicrobial resistance, allowing bacterial species to thrive in unfavorable conditions through the establishment of the matrix, and at the same time, is a hotspot of sharing of antibiotic resistance genes through horizontal transfer due to the close association of bacterial groups. This opens up research for mucus extracts to be used as biofilm disruptors as well.
On the aspect of food microbiology, spoilage is a problem in the fishery products industry due to handling and transport conditions across the supply chain, and typically, spoilage is characterized by a succession of spoilage microbial species. Motivated by the biocontrol activity of the fish mucus, Leng et al. (2022) tested the activity of the skin mucus of the snakehead fish against E. coli and its possible application in the preservation of refrigerated fish fillets (43). Positive results were found with the reduced viable bacterial count in the fillets and improved organoleptic properties for those coated with fish mucus. This extends the possible application of fish mucus as spoilage control agents as well.
In addition, Wang and colleagues worked on the antibacterial activity, enzyme production, and antiparasitic activity of the skin mucus of the yellowtail clownfish (Amphiprion clarkii)(44). Potent activity against ubiquitous fish pathogens, A. hydrophila and V. parahaemolyticus were observed, but no activity against the Gram-positive indicator strains. The mucus had high peroxidase, lysozyme, and protease contents. Such enzymes are integral agents that kill pathogens. There was also apparent time-dependent and dose-dependent antiparasitic activity against the protozoan parasite Cryptocaryon irritans. Fish mucus might also confer protection for the fish against parasitic organisms.
Pushing further the bioactive potential of the fish mucus, Vennila et al. (2011) and Fuochi et al. (2017) studied the mucus of the common stingray (Dasyatis pastinaca) and cowtail stingray (Daysatis sephen) (45, 46). Both mucus samples demonstrated greater activity against Gram-negative indicator strains. Apart from the antimicrobial activity, Vennila et al. (2011) found out about the serine and metalloproteases in the samples with proteolytic activity, which, in part, may be responsible for the antimicrobial activity as a mode of action. Additionally, Fuochi et al. (2017) tested the antiproliferative properties of the skin mucus motivated by the increasing resistance to chemotherapy treatments among cancer patients. In their research, the mucus was toxic to acute leukemia cells with no observed effects on myeloma and neuroblastoma cells, providing a glimpse of the potential of fish mucus in cancer research. The studies discussed in this section are summarized in Table 1.
Table 1.
Inhibitory properties of mucus from various fish species
| Fish Species | Mucosal Surface | Bioactivity | References |
|---|---|---|---|
| Common eel (Anguilla anguilla) | Skin | Greatest inhibition against S. paratyphi | (11) |
| Skin | Inhibited A. awamori and C. falcatum | (35) | |
| Snakehead fish (Channa sp.) | Skin and gut | Most inhibition against V. fischeri and P. aeruginosa | (30) |
| Skin | Inhibited growth of K. pneumoniae, P. aeruginosa, B. subtilis, and A. hydrophila | (32) | |
| Skin | Greatest inhibition against V. cholera | (31) | |
| Skin | Inhibited E. coli and growth of viable bacteria in refrigerated fish fillets | (43) | |
| Skin | Maximum activity against E. coli, S. aureus, and H. gramineum | (33) | |
| Indian carp (Catla catla) | Skin | Inhibited A. awamori and C. falcatum | (35) |
| Skin | Inhibited various bacterial pathogens | (38) | |
| mrigal (Cirrhinus mrigala) | Skin | Inhibited A. awamori and C. falcatum | (35) |
| Skin | Inhibited various bacterial pathogens | (38) | |
| Swamp eel (Monopterus albus) | Skin | Greatest inhibition against Fusarium spp. | (36) |
| Skin | Decreased growth of A. niger and M. gypseum | (37) | |
| Skin (formulated into topical gel) | Complete recovery of mice after intracutaneous injection with S. pyogenes, S. aureus, M. gypsum, C. albicans | (41) | |
| Pool barb (Puntius sophore) | Skin | Substantial antagonistic activity against E. coli, P. aeruginosa, B. subtilis, and S. aureus; antiadhesion and antibiofilm properties | (42) |
| Tilapia (Oreochromis spp.) | Skin | Inhibited E. coli, S. aureus, B. subtilis, B. cereus, B. megaterium, A. flavus, and C. albicans | (40) |
| Rohu (Labeo rohita), Grass carp (Ctenopharyngodon idella), and Silver carp (Hypophthalmichthys molitrix) | Skin | Inhibited various bacterial pathogens | (38) |
| Book trout (Salvelinus fontinalis) | Skin | Lowest MBC against S. enterica | (28) |
| Haddock (Melanogrammus aeglefinus) | Skin | Lowest MBC against E. coli, S. enterica, and Y. ruckeri | (28) |
| Hagfish (Myxine glutinosa) | Skin | Lowest MBC E. coli and Y. ruckeri | (28) |
| Common stingray (Dasyatis pastinaca) | Skin | Inhibited E.coli, P. aeruginosa, and K. pneumoniae and antiproliferative against acute leukemia cells | (46) |
| Cowtail stingray (Dasyatis sephen) and Whitespotted whipray (Himantura gerrardi) | Skin | Inhibited various pathogens and showed protease activity | (45) |
| Chame fish (Dormitator latifrons) | Skin |
Inhibited Bacillus sp. V. harveyi V. anguillarum, and V. vulnificus | (29) |
|
Tilapia (Oreochromis niloticus), Catfish (Clarias batrachus), Snakehead fish (Channa striata) | Skin | Inhibited E. faecalis, S. aureus, M. luteus, K. pneumoniae, P. aeruginosa, A. hydrophila, E. coli, S. marcescens | (34) |
| Yellowtail clownfish (Amphiprion clarkii) | Skin | Antiparasitic activity against C. irritans; antibacterial activity, and enzyme production | (44) |
|
catla (Catla catla) rohu (Labeo rohita) silver carp (Hypophthalmichthys molitrix) grass carp (Ctenopharyngodon idella) | skin | Indigenous species had greater antimicrobial activity than exotic species | (39) |
These studies are evidential of the potential of the fish mucus for bioprospecting of other bioactivities with applications in fish and human health, even extending to medicine research. Research work on the bioactive properties, thus far, utilized the skin mucus due to its accessibility and non-invasive sampling methods. It can be collected without having to kill the fish. There are gaps with regards to evaluating the bioactive properties of the mucosa found in other organs, such as the gills and nasal cavity, which, from a physiological standpoint, assume a different function, and the protection conferred by the mucosa in those parts may be specialized and unique. For instance, some pathogens, such as Vibrio anguillarum and Aeromonas salmonicida use the gills as one of their main entry points in fish (7). The gill mucosa must have components that are able to counteract these pathogens, and such components may or may not be found in other mucosal layers.
It can also be observed that those often studied for their mucosal secretions are relevant fish species in aquaculture as a more conventional approach to investigating mucosal immunity. Some of these farmed species, such as the tilapia, snakehead fish, and the swamp eel, however, have become invasive to habitats they are introduced into, and in part, this can be due to their biological advantages, which are some of the characteristics desired for aquaculture production. In retrospect, the invasive success of these fish in colonizing new habitats is also indicative of a robust innate immunity to counteract threats in these non-native environments (47). Thus, mucosal studies can also be geared toward the valorization of invasive species as potential subjects on account of their physiological edge over other species.
BIOACTIVE COMPOUNDS FROM THE MUCUS
The defensive properties of the mucosa can be attributed to the bioactive molecules either inherent in the mucus or produced by commensal strains. The exploration of commensal strains as probiotic candidates is discussed in the latter portion of the paper. As presented in the previous section, mucosal extracts have broad activity against the tested pathogens, which is not surprising considering that it is part of innate immunity. To narrow down such activity to particular components in the mucus, there have been studies on the isolation, characterization, and evaluation of various compounds that contribute to the protective properties of the mucosal barrier as extensions of their established bioactive properties in earlier pursuits. With the advent of more advanced molecular technologies, the number of studies on these specific molecules has increased in recent years (Table 2). These biomolecules range from antimicrobial peptides (AMPs), lectins, proteins, mucins, and several others and have stark differences in their modes of action. The presence of these protective molecules is consequential of the coevolution between host and pathogens in the aquatic environment. Characterization efforts, thus far, have elucidated that several of these biomolecules have high similarity with homologs present in terrestrial organisms, with even higher activity than their terrestrial counterparts necessitated by the constant contact of aquatic organisms to the water environment (48). Additionally, isolated biomolecules of aquatic origin are more stable, unlike several terrestrial biomolecules that are less stable in extreme environments such as marine water with fluctuating salinities. It is posited that the mucus, with its immune function in fish, is a rich source of potent biomolecules that can be used for therapeutics in fish and humans. With fewer explorations in aquatic habitats, the biomolecules isolated are rather novel and uncharacterized.
Table 2.
Bioactive substances from the mucus of various fish species
| Fish Species | Mucosal Surface | Bioactive molecule | References |
|---|---|---|---|
| Hagfish (Myxine glutinosa) | skin | Myxinidin antimicrobial peptide | (50) |
| Atlantic salmon (Salmo salar) | skin | NK-lysin | (51) |
| Pufferfish (Takifugu pardalis) | skin | Novel hepcidin type 2-like antimicrobial peptide | (48) |
| African catfish (Clarias gariepinus) | skin | glycoprotein | (53) |
| Japanese eel (Anguilla japonica) | skin | AJN-10 antibacterial peptide | (49) |
| Catfish | skin | CF-14 antimicrobial peptide | (54) |
| Tench (Tinca tinca), eel (Anguilla anguilla), and rainbow trout (Oncorhynchus mykiss) | skin | Pore-forming antibacterial glycoproteins | (49) |
| Climbing perch (Anabas testudineus) | skin | ATMPs | (55), (56) |
One of the pioneering studies on bioactive molecules from the fish mucosa was conducted by Ebran et al. (2000) on novel glycoproteins from three fish species: the tench (Tinca tinca), eel (Anguilla anguilla), and rainbow trout (Oncorhynchus mykiss) (49). Pore-forming was the proposed mechanism for the activity of the glycoproteins correlated with potent antibacterial activity. Subramanian et al. (2009) were also able to isolate myxinidin antimicrobial peptide, which was found to inhibit several pathogens without having hemolytic effects against mammalian red blood cells (50).
In the study of Valero et al. (2019), blood serum and skin mucus samples from the Atlantic salmon (Salmo salar) were collected (51). An antimicrobial peptide, NK-lysin, is a known bactericide in teleost tissues. However, in this study, its concentration and bactericidal properties are more pronounced in the skin mucus samples than in the sera when tested against indicator pathogens. It was the first documentation of the NK-lysin in the skin mucus and its greater concentration in the skin mucus was indicative of greater antimicrobial activity.
Similarly, studies wanted to determine the upregulation of certain mucosal molecules when the fish is exposed to pathogens or stress. Go et al. (2019) tested the expression of a novel hepcidin, HAMP2, purified from the skin mucus of pufferfish (Takifugu pardalis), where it presented potent antimicrobial activity and was upregulated after pathogenic challenge with Vibrio anguillarum, indicating its essential role when the fish is exposed to pathogens from the environment (48). Similarly, Liang et al. (2011) exposed Japanese eels (Anguilla japonica) to injections of Aeromonas hydrophila prior to collection of skin mucus, where they were able to isolate and purify a novel antimicrobial peptide, AJN-10, with little similarity to proteins in the database (52). It was the first inducible peptide to be isolated from the mucus of the Japanese eel with potent activity.
As a source of novel antimicrobial compounds, a number of researchers have evaluated the individual and synergistic activity of mucosal compounds against existing antimicrobials. For instance, Abdel-Shafi et al. (2019) extracted glycoprotein from the skin mucus of the African catfish (Clarias gariepinus), which had broad activity against various microbial pathogens (53). In their study, the catfish glycoprotein had synergistic activity when combined with existing antibiotics, such as chloramphenicol, with a general trend of increasing antibacterial action observed with increasing glycoprotein proportion up to a certain threshold. Li et al. (2019) also characterized an antimicrobial peptide from catfish mucus, CF-14, and reported broad antimicrobial activity, notably on Shewanella putrefaciens, a spoilage bacterial species (54). Based on the assays conducted, it is proposed that CF-14 binds to DNA in the cell rather than the membrane.
Aside from compounds with antimicrobial properties, some peptides from the fish mucus have shown promise with anticancer activity. In the connected research work of Najm et al. (2021) and Law et al. (2023), antimicrobial peptides from the skin mucus of the climbing perch (Anabas testudineus) were reported to arrest breast cancer growth through induction of apoptosis and interaction with apoptosis-related genes (55, 56). This is a huge leap for mucosa-derived molecules from antimicrobial to anticancer activities. This fortifies the claim that there should be more bioprospecting research to be conducted on fish mucus as a novel source of bioactive molecules. With the advent of various -omics technologies, the disparities in the studies on relevant mucosal biomolecules can be addressed (12, 25). As an underexplored source with inherent immune function, the mucosa has the potential to augment lapses in the pipeline for the search for new antibiotics to address the global crisis of antimicrobial resistance (14, 57).
POTENTIAL PROBIONTS FROM THE MUCOSA
The mucosal microbiota has immense contributions to fish health. It varies between environments, species, and even different tissues in the same fish (58, 59); but there are species that comprise the core microbiota that is consistent regardless (19, 24, 60). As part of the core microbiota, they have purported contributions to the host, not only in immunity but also in other physiological functions, such as growth, metabolism, and nutrient uptake (47, 61). The core species include commensal strains that are maintained by the mucus due to their indispensable benefits for the host fish. This makes them candidate probionts as viable and sustainable alternatives for synthetic antimicrobials used in aquaculture. The intimate association of the mucosal layer with the fish has implications for fish health- a perspective understudied (3, 62).
The mucosa, as an immune organ, aims to deflect pathogenic species but at the same time, preserve commensal strains that have pivotal roles in orchestrating immune responses (7, 17, 24). Mucosal-associated lymphoid tissues (MALT) have displayed mechanisms that allow them to discriminate between pathogenic and non-pathogenic mucosal bacteria (23, 24, 63). Host immune systems and commensal bacteria have co-existed for some time, sharing a bidirectional regulation (64). Some commensal strains induce the immune system to upregulate factors that are integral in the immune response against potential invaders. On the other hand, some immune factors have been conditioned to maintain, if not increase, the populations of these commensal bacterial species in the mucosa through cascades of recognition processes (24, 61, 65, 66). As to the mechanisms of how commensal strains interplay in mediating immune response, they are yet to be fully understood. Proposed hypotheses include interference in the adhesion of pathogenic bacteria in the host, hindering their infection routes, or the commensal strains producing antagonistic metabolites that can either kill pathogens or intervene with their modes of action (7, 8, 67, 68, 69, 70). In support of the first proposition, for instance, in the study of Vine et al. (2004), using isotope probes, probiotics isolated from clownfish gut were checked for their competitive success against aquatic pathogens, A. hydrophila and V. alginolyticus as far as attachment to the mucosal matrix is concerned (67). When the probiotics were added after pathogen settlement, the attachment of the latter was reduced. This implies that the probiotic is able to displace the initial pathogen, and growth and subsequent infection caused by the pathogen may be mitigated under the presence of the probiont. Similarly, Chabrillon et al. (2006) investigated the interference of probiotic candidates from the gilthead seabream against Listonella anguillarum (68). Under exclusion, competition, and displacement assays, some probiotic candidate was subjected to challenge tests. Overall, the probiotic interfered with the growth of the pathogen and was able to reduce the mortality rate of the fish.
Research on screening the mucosal microbiome in search of commensal strains that can benefit fish health has increased in number over the past years as an extension of the bioactive properties of the mucosal extracts and the isolated bioactive molecules from it. Probionts have been proven to provide benefits such as antagonism against pathogens, whether through exclusion, competition, or displacement mechanisms, immunomodulation of responses, and improvement in health, typically through the restoration of the homeostasis of the mucosal microbiome (7, 68, 71). The definition of probiotics has diversified in recent years to accommodate some concerns in aquaculture. Conventionally, probiotics encompass live microorganisms that are supplemented into feeds to benefit the host, enhancing microbial homeostasis (7). However, such a definition is unable to cover some specific cases, resulting in a revised definition- probiotics can be live or dead or can be a component of a microbe that functions with different modes of action and generally provides benefit for the host or its environment. This is able to address concerns regarding the administration of probiotics, where some probiotics are added to rearing water instead of feeding to fish; some probionts having multifaceted benefits apart from homeostasis maintenance; and the viability of the microorganism during administration, where some microbes are killed prior to use.
While there is a robust repository of studies on probionts from fish, especially those isolated from gut tissue, there are remote studies on the potential probionts from the mucus, which, as established previously, is comprised of a different community of bacterial strains than the surrounding environment. Of those studies that worked on mucosal probionts, most of them have only worked on establishing the antagonistic activity and some probiotic characteristics, and the niche for those that extend to in vivo characterization is yet to be fully explored. These studies are summarized in Table 3. There exists a wide roster of these commensal bacteria that have probiotic potential and application, evaluated using in vitro and in vivo assays, some proven to decrease mortality in fish subjected to pathogenic challenge tests. Bacterial genera commonly sourced for probionts include Bacillus, Carnobacterium, Lactobacillus, Aeromonas, Alteromonas, Pseudomonas, and Vibrio, to name a few(24, 61).
Table 3.
Probiotic candidates isolated from the mucosal surfaces of various fish species
| Fish Species | Mucosal Source | Probiotic Candidate | Probiotic Properties | References |
|---|---|---|---|---|
|
Indian goat fish (Parupenus indicus) | skin | Pseudoalteromonas sp. | Scored highest based on probiotic characteristics testing | (71) |
|
Bighead catfish (Clarias macrocephalus) | skin | Acinetobacter pittii | Antagonism against marine and freshwater pathogens | (8) |
|
Freshwater Atlantic stingray (Dasyatis sabina) | skin | Bacillus cereus | Broad spectrum activity against MRSA, MSSA, VRE, and E. coli | (72) |
|
Marine Atlantic stingray (Dasyatis sabina) | skin | Photobacterium sp. Vibrio sp. | Inhibited B. subtilis | (72) |
|
Cownose ray (Rhinoptera bonasus) | skin | Halomonas sp. | Inhibited MRSA (>10 mm) | (72) |
| Bacillus sp | Inhibited MRSA (7.5 mm), MSSA (8.5 mm), B. subtilis (10 mm) | |||
| Shewanella sp. | Inhibited MSSA (>10 mm) | |||
| Alteromonas sp. | Inhibited MSSA (>10 mm) | |||
|
Atlantic devil ray (Mobula hypostoma) | skin | Vibrio sp. Pseudoalteromonas sp. Alteromonas sp. | Inhibited MRSA, B. subtilis, V. shilonii | (72) |
|
Rainbow trout (Oncorhynchus mykiss) | Skin | A. stackebrandtii A. psychrolactophilus | Inhibited M. hiemalis | (23) |
| P. maritimus A. psychrolactophilus | Inhibited S. australis | (23) | ||
| Gut | B. amyloliquefaciens Paenibacillus spp | Inhibited Y. ruckeri strains | (73) | |
|
Labeo fish (Labeo calbasu) | Skin | B. cereus | Improved growth, immunity, and survival of L. calbasu | (74) |
In the study of Thelma and Asha Devi (2016), the skin mucus of the Indian goat fish (Parupenus indicus) was screened for epibiotic bacteria with probiotic potential (71). Parameters such as acid and bile tolerance, antibiotic resistance, antagonism against indicator pathogens, and tolerance to varying pH, heat, salt, and bile concentrations were scored. Some Pseudoalteromonas sp. garnered the highest probiotic potential based on the set criteria and were recommended to be subjected to further characterization.
Meanwhile, In the study of Bunnoy et al. (2019), the probiotic isolate highly similar to Acinetobacter pittii from the skin mucus of the bighead catfish (Clarias macrocephalus) was the first probiotic candidate from the genus Acinetobacter and is believed to be safe for use as alternatives for antibiotics in catfish farming (8). The isolate displayed antagonism against various freshwater and marine pathogens.
Ritchie et al. (2017) conducted an extensive survey of the skin mucus of different fish species of marine rays and skates for antibiotic-producing bacteria (72). Sharks and rays are also able to produce epidermal mucus but are not easily extracted due to denticles present on the epidermis. Nonetheless, the researchers conducted an evaluation to identify bacteria that contribute to protection from infection brought about by wounds. The antibacterial activity against different pathogens was measured in terms of zones of inhibition (ZOI). Out of the 1,860 bacterial isolates screened, 311, or 16.7%, showed activity against indicator pathogens. From the freshwater Atlantic stingray (Dasyatis sabina), a Bacillus cereus isolate demonstrated broad-spectrum activity against methicillin-resistant Staphylococcus aureus (MRSA), methicillin-sensitive Staphylococcus aureus (MSSA), vancomycin-resistant Enterococcus (VRE), and E. coli. From the marine Atlantic stingray, Photobacterium sp. and Vibrio sp. inhibited the growth of Bacillus subtilis. The cownose ray (Rhinoptera bonasus) had the most isolates with significant antibacterial activity against several pathogens. Some Halomonas sp. had a remarkable zone of inhibition greater than 10 mm against MRSA. A Bacillus species inhibited MRSA, MSSA, and Bacillus subtilis. Some Shewanella and Alteromonas isolates had a zone of inhibition greater than 10 mm against MSSA. The Atlantic devil ray (Mobula hypostoma) skin mucus also harbored Vibrio sp., Pseudoalteromonas sp., and Alteromonas sp. that were found to inhibit MRSA, B. subtilis, and V. shilonii.
The rainbow trout (Oncorhynchus mykiss) was also explored by Lowrey et al. (2015) for symbiotic groups that are able to inhibit important aquatic fungal pathogens, Mucor hiemalis and Saprolegnia australis (23). Putative Arthrobacter stackebrandtii and Athrobacter psychrolactophilus inhibited M. hiemalis in the qualitative coculture assays. Psychrobacter maritimus and A. psychrolactophilus isolates inhibited S. australis. With most studies on probiotics conducted on gut tissue, the study of Medina et al. (2020) was able to localize the intestinal mucus secretion to isolate some probiotics (73). Tested against Yersinia ruckeri, some Paenibacillus spp. and Bacillus amyloliquefaciens were isolated and displayed probiotic potential in preliminary tests such as in vitro antagonism, tolerance to bile salts and pH, and hydrophobicity.
Thus far, the enumerated studies focused on the individual effect of different mucosal probionts. While the abovementioned studies extrapolated the probiotic potential with certain properties as indicators, in vivo tests are still needed to substantiate. In the study of Bhatnagar and Rathi (2023), Bacillus cereus was isolated from the skin of the labeo fish (Labeo calbasu) and administered with another autochthonous probiotics, Aneurinibacillus aneurinilyticus to the intestine (74). The synergy of both probionts improved the overall health of the fish through stimulation of the immune system. This opens up research on probiotic cocktails in order to magnify the desired effects on fish for disease prevention and control.
Probiotics are typically selected based on their antagonistic activity against target pathogens and adhesion to epithelial mucus, with the second property bypassed by the mucosal probionts since they are already known to adhere and thrive in it (68, 74, 75, 76, 77). Such studies collectively suggest that the mucus can harbor potential probionts that can be harnessed for aquaculture applications with the requisite to elaborately characterize the isolates (8, 71, 78). Future studies can explore such mucosal surfaces to unveil candidate commensal strains that have significant contributions to immunity, whether systemic or localized in the tissue. Sequencing efforts to identify these probionts should be performed to widen the array of commensal strains for potential utilization in aquaculture. From an evolutionary lens, some commensal species might even be shared among taxa and retained over the course of evolutionary time.
To elucidate the roles of commensal microbiota within fish mucus, omics-based approaches can be employed to investigate host-microbiota interactions and their implications for fish health (24, 59). While previous research has predominantly focused on skin and gut mucosal communities, further investigation is needed to characterize the microbiomes of gill, buccal region, and pharyngeal mucosa (7, 22, 24, 79, 80). Additionally, expanding research beyond freshwater species to encompass marine fish is crucial (4, 18, 71), despite the inherent sampling challenges associated with marine environments. Given the established discovery of antimicrobial compounds from marine invertebrates and sediments, the mucus of marine fish presents a promising reservoir for novel bioactive molecules.
CONCLUSION
The inherent protective properties of fish mucus suggest its potential for disease management in aquaculture. Converging evidence from existing studies demonstrating bioactivity, the presence of bioactive molecules, and potential probiotic candidates underscores the need for further research into fish mucus as an underexplored resource. This research could contribute to addressing the critical challenges of antimicrobial resistance and disease outbreaks in aquaculture. Advancements in sequencing and omics technologies can accelerate the discovery of novel bioactive compounds and bacterial strains within this complex biological matrix. The mucus can be subjected to modern sequencing techniques to attain a better understanding of the interactions in the mucosa with respect to preventing the disarray of the microbiota essential in disease, and at the same time, utilize it as a biomonitoring tool of the environment, such as the surveillance of pollutants. Mucosal delivery can be used as an alternative route for the administration of probiotics considering their close association with the host and inherent immune contribution. Additionally, since the mucosa is a dynamic organ in fish affected by environmental conditions and varies among host species, the variation in ecological niches is also tantamount to a wider reservoir of potential biomolecules and probionts for extensive bioprospecting.