INTRODUCTION
Bacteremia is defined as the presence of bacteria in the blood, which is revealed by isolating bacteria from blood cultures (1).
The source of bacteremia can be diverse depending on the patient’s clinical characteristics. In nephrological pathology, their incidence is mainly correlated with the increased use of central or peripheral venous catheters, which is unavoidable in hemodialysis (2).
Infectious complications are the second leading cause of morbidity and mortality in dialysis patients after cardiovascular events. According to the results of a study conducted in the USA on patients under dialysis, the cumulative annual incidence of infection-related hospitalization was 26% for children and 31% for adults (3).
In Burkina Faso, the prevalence of Catheter-Related Infections in patients under dialysis is 25%.
Historically, the most common pathogens encountered in hemodialysis patients have been Gram positive cocci, mainly Staphylococcus aureus followed by coagulase negative staphylococci (4).
In addition, Gram-negative bacteria have been identified less frequently, especially during outbreaks (5).
However, given the increased use of antibiotics in this particular subset of patients and their recurrent exposure to the healthcare system, there has been a rise in the incidence of infections with multidrug resistant (MDR) organisms (6).
Thus, identification of locally prevalent pathogens is important for optimal care in patients since the choice of empiric treatment or prophylactic antibiotics depends on the prevalent pathogens in a local community.
Unfortunately, there is a paucity of such information in Senegal especially in Dakar.
The aim of this study is to determine the etiology of bacteremia among hemodialysis patients and to determine their resistance profiles.
MATERIAL AND METHODS
Study design and population
This is a retrospective study of patients undergoing chronic hemodialysis at nephrology department of CHNU Aristide Le Dantec hospital between January and December 2021.
Inclusion criteria
All patients, regardless of sex or age, hospitalized in the Nephrology Department during the study period and diagnosed with bacteremia were included in this study.
Sampling
We received blood culture balloons from the Nephrology Department of CHNU Aristide Le Dantec. They were then immediately incubated in the Bactec FX 40 after verification of their conformity.
Microorganism’s identification
With the detection of an alarm due to growing suspicion of microorganisms, we performed a Gram stain, and then inoculated with the appropriate media culture for the identification of the suspected germ.
After 5 days when we have not alarm from the Bactec FX 40, we inoculated cooked blood agar before giving negative results.
When media culture was positive, microorganisms were identified using morphological, cultural, biochemical or antigenic characters using respectively microscopy, media culture, API tests or rapid tests.
Antibiotic susceptibility testing
The antimicrobial susceptibility was performed using antibiotics standard disk diffusion method (Oxoid Ltd, Basingstoke, Hampshire, UK) including β-lactams (ampicillin 10 μg, amoxicillin 20 μg, amoxicillin/ clavulanic acid 20/10μg piperacillin 30 μg, ticarcillin 75μg, cefepim 30μg, cefexim 5μg , cefoxitin 30μg, imipenem 10 μg), furoquinolones (acide nalidixic 30 μg, norfloxacin 10 μg, ciprofloxacin 5 μg), aminoglycosides (amikacin 30 μg, gentamycin 10 μg, tobramycin 10 μg) and fosfomycin for Gram negative bacteria
For Gram positive bacteria antibiotics such as β-lactams (penicillin 1U, oxacillin 5 μg) furoquinolones (norfloxacin 10 μg), aminoglycosides (amikacin 30 μg, gentamycin 10 μg, tobramycin 10 μg), glycopeptid (vancomycin 30μg), cyclins (tetracyclin 30μg, tigecyclin 15μg), Triméthoprim/sulfaméthoxazol 1,25/23,75 using antibiotics standard disk diffusion method ( (Oxoid Ltd, Basingstoke, Hampshire, UK) according EUCAST 2021 (7).
Data analysis
The WHONET software (version 5.6) was used to analyze the antimicrobial susceptibility test results. Mean values and standard deviation for diameter of inhibition zones, and geometric mean MICs were calculated. The results were expressed as mean values ± SD or as geometric means. In addition, Excel software 2010 was used.
RESULTS
Frequency of bacteremia
70 blood cultures from the Nephrology Department were studied during the study period. Among these blood cultures, 37 were positive (53%), 04 were considered contaminated (6%) and 29 were negative (41%) (Fig. 1).
Distribution of bacteremia by age group
The mean age of patients with bacteremia was 33 years old with extremes of 19 and 89 years old.
22% of patients were between 0 and 30 years old, 51% were between 31 and 59 old and 22% were more than 60 years old (Fig. 2).
Distribution of bacteremia by sex
Among these 37 positive blood cultures, 20 (54%) were from women and 17 (46%) from men with 0.85 sex ratio (Fig. 3).
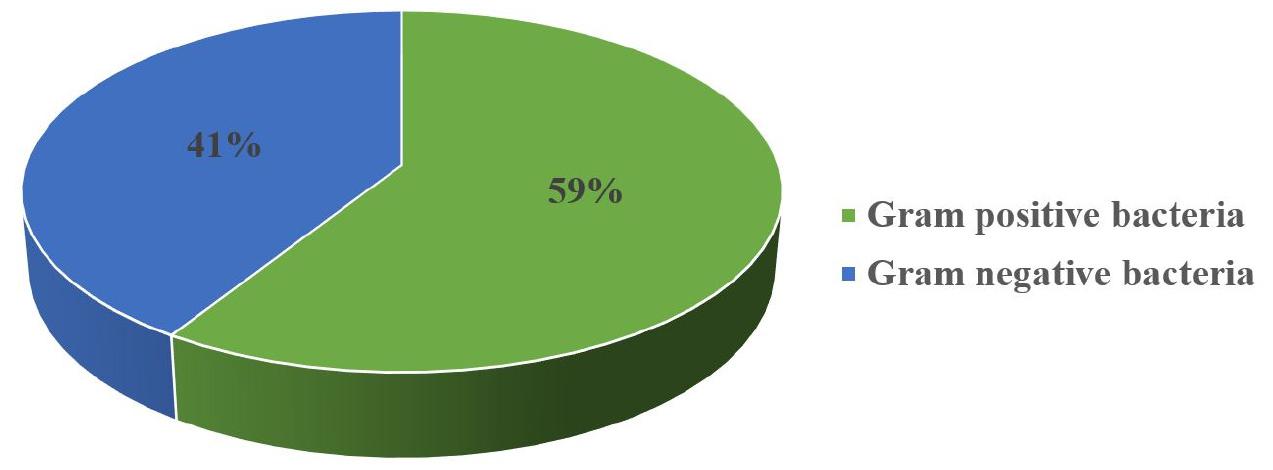
Distribution of bacteria in bacteremia
Among bacteria isolated from blood cultures, Gram negative bacteria are most important with 59% (Fig. 4).
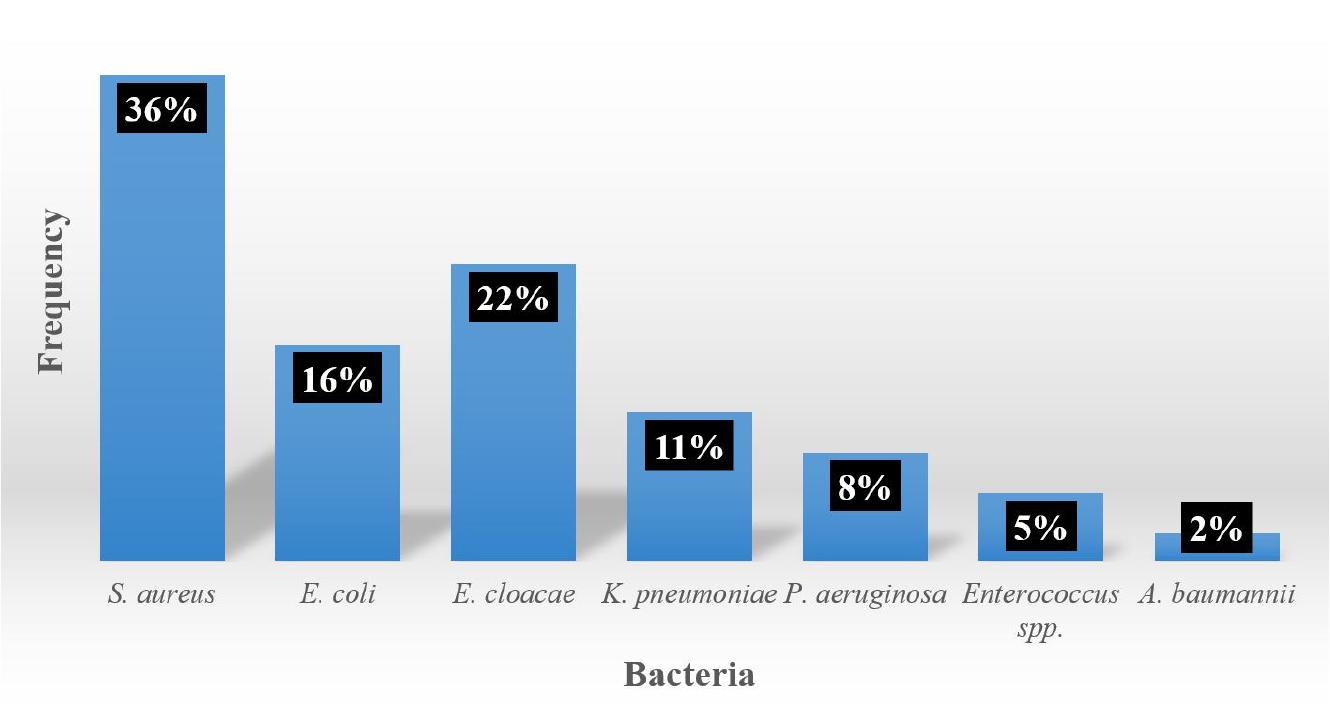
Distribution of germs in bacteremia
Among bacteria responsible of bacteremia, Staphylococcus aureus (n=13; 36%) were most important followed by Enterobacter cloacae (n=8; 22%), Escherichia coli (n=6; 16%) and Klebsiella pneumoniae (n=4; 11%) (Fig. 5).
Antibiotics susceptibility of Staphylococcus aureus
Most active antibiotics were oxacillin (83%), tobramycin (92%), gentamycin (100%) and vancomycin (100%). However, all S. aureus strains were resistant to norfloxacin (Fig. 6).
Antibiotics susceptibility of Enterobacter cloacae
About E. cloacae, antibiotics such as cefepim (88%), cefixim (75%), tobramycin (88%), imipenem (100%) and tigecyclin (100%) were most active.
However, fosfomycin was not active against E. cloacae (Fig. 7).
Antibiotics susceptibility of Escherichia coli
Antibiotics such as imipenem (100%), ceftazidim (83%), cefoxitin (83%), and amikacin (100%) gentamicin (83%) were most active (Fig. 8).
Antibiotics susceptibility of Klebsiella pneumoniae
Antibiotics such as imipenem (100%) and ciprofloxacin (100%) were most active. However, tobramycin was not active against K. pneumoniae (Fig. 9).
DISCUSSION
The importance of blood cultures in detecting bacteremia is well known. Numerous studies showed the interest of this subject (8, 9).
In our study positivity rate of blood, culture was 53%. Studies carried out in Togo and Burkina Faso showed a positivity blood cultures rate of 40% and 44.5% respectively Results found in our study were close to than found in Burkina Faso (10).
However, results from India and Ethiopia about blood cultures showed low rates of positivity with 16.5% and 15.5% respectively (11, 12). These differences may be linked to the improved quality of the technical platform with the use of automated blood culture machines.
Our study showed a female predominance (54%) in bacteremia with 0.85 sex ratio. This result was close to those carried out in Dakar in 2018 (56%; M/F: 0.79) and in Brazzaville in 2014 (57.62%; M/F: 1.36) (13, 14). However other studies carried out respectively in Ivory Coast and India showed the predominance of men in bacteremia with 1.87 and 1.23 sex ratio respectively (15, 16).
In our study bacteremia were more frequent in the 31-59 age group. However, in Burkina Faso, the 06-35 age group was most frequent (12, 17).
This difference could be explained by the fact that most of the patients hospitalized in the Nephrology department in our country were adults.
Also in our study about bacteria isolated in bacteremia, Gram negative were most frequent (59%). Studies carried out in Tunisia, Bamako, Burkina Faso and Togo showed Gram-negative predominance in bacteremia with 72.3%, 59.8%, 80% and 57.9% respectively. (2, 17, (18, 19). However, studies carried out in Japon and India showed Gram-positive bacteria in bacteremia with 56.02% and 62.37% (9, 11).
Among bacteria identified in our study, the most frequent were S. aureus (36%), E. cloacae (22%), E. coli (16%) and K. pneumoniae (11%).
Regarding antibiotics susceptibility profile of S. aureus methicillin activity was 83%. Similar results were found in Maroco and France with de 93.8%, 88.8% respectively (20, 21). However, in Ethiopia a low rate of methicillin susceptibility (60%) was found (12).
In addition, antibiotics such as gentamycin (100%), tobramycin (92%) and vancomycin (100%) were active against S. aureus. Similar results were found in Maroco with an activity of 80% for gentamicin, 80% for tobramycin and 100% for vancomycin (22).
Regarding antibiotics susceptibility profile of E. cloacae in our study, antibiotics such as cefepim, imipenem, tobramycin and tigécyclin were most active. Similar results were found in Maroco (23).
In addition, in our study with E. coli strains antibiotics such as amikacin (100%) gentamicin (83%) imipenem (100%) and ceftazidim (83%) were active. Previous studies in Dakar showed similar results (24).
In conclusion, this study shows the high rate of bacteremia in nephrology department of CHNU Aristide Le Dantec with antibiotics more or less active against bacteria such as E. coli,Enterobacter cloacae, K. pneumoniae and S. aureus.
For better management of bacteremia in that department, we need to implement a systematic surveillance program. In addition, we need to sensitize all medical and paramedical staffs in that department in order.