INTRODUCTION
Ticks are obligate blood-feeders that solely thrive on the blood of mammals, birds, and occasionally reptiles and amphibians for their nutritional requirements, growth and propagation. Ticks are successors to mosquitoes, in the transmission of a variety of pathogens to humans and animals (1).
Apart from harboring the pathogens of public health and veterinary importance, ticks were reported to have two groups of endosymbionts. The first group is an obligate mutualistic symbiont which is essential for the ticks’ survival and growth and provides nutritional supplements such as vitamins. These obligate endosymbionts are transovarially transmitted, for example, Coxiella-like endosymbionts(CLEs) and Francisella-like endosymbionts (FLEs) (2). CLEs in Ambylomma americanum contribute to the biosynthesis of several vitamins such as folic acid (Vit B9), riboflavin (Vit B2), pantothenic acid (Vit B5), thiamine (Vit B1), and biotin (Vit B7), all of which are essential for the fitness, reproduction, and survival of the ticks. The second group includes the facultative endosymbionts that do not affect the survival, but augment the physiology and reproduction in ticks (e.g., Midichloria sp., Arsenophonus sp., Rickettsiella sp., and Wolbachia sp (3, 4, 5). Elimination of CLEs using antibiotics conferred detrimental effects on vector fitness, survival, and vector competence. For instance, oxytetracycline exposure in Amblyomma americanum resulted in a significantly prolonged duration of oviposition and a lower number of viable larvae per tick (6). Very limited studies were carried out to explore the endosymbionts diversity in the ticks infesting the domestic animals in India. Hence, this preliminary study was carried out to explore the commonly prevalent endosymbionts in the ectoparasites infesting the pets and livestock in Puducherry.
MATERIALS AND METHODS
This exploratory study was carried out in the Puducherry region of the Union Territory of Puducherry for 2 months (August-September 2022). The institutional Animal Ethics Committee approved the study (IAEC/ICMR-VCRC/2022-B/2). The domestic animals brought to 11 veterinary dispensaries were examined for ectoparasite infestation and the ectoparasites were retrieved using fine tweezers and transferred to a labeled vial containing 80% ethyl alcohol.
Taxonomical identification of the ectoparasites
The ectoparasite specimens were identified based on the morphological features under a stereo microscope using the taxonomical keys (7). Among the ectoparasites collected, only ticks were subjected to molecular taxonomy and endosymbiont screening by PCR.
DNA isolation and molecular confirmation of the ectoparasites
The taxonomically similar species of ticks were pooled based on the geographical location of collection, sex, life stage, and animal hosts. From the above pools, 2 representative specimens from each species of ticks were used for DNA extraction. The tick specimens were surface sterilized using 1% bleach followed by 70% ethyl alcohol and DNA was extracted following the published protocol (1). The quantity and quality of the DNA were analyzed using a spectrophotometer (Nanodrop, Thermo Scientific, Wilmington, USA). Confirmation of the taxonomically identified ectoparasites was achieved by PCR amplification and nucleotide sequencing of the genes such as cytochrome oxidase subunit I (COI), 16S rRNA, and 18S rRNA (1).
Demonstration of transovarial transmission of endosymbionts in ticks
To confirm the vertical transmission of the endosymbionts, two full-fed adult females of R. sanguineus infesting a dog were collected and allowed to oviposit under laboratory conditions (28℃ and 80% relative humidity). Seven days post oviposition, 50 eggs from each egg mass were collected and pooled (n = 2). The remaining eggs were incubated for the emergence of larvae. The emerged larva was randomly selected and made into 6 pools (10 larvae constituted a pool). The eggs and larva were surface sterilized, homogenized and DNA was extracted as mentioned earlier (1).
Molecular identification of the endosymbionts in the field-collected ticks, eggs and larvae raised in the laboratory
The presence of Midichloria mitochondrii, Wolbachia, Coxiella-like and Francisella-like endosymbionts in the DNA of the field-collected ticks, eggs, and larvae were screened by PCR (8, 9, 10, 11). The PCR products were resolved on a 1.2% agarose gel by electrophoresis.
Nucleotide sequencing and analysis
The PCR products from the positive samples were subjected to Sanger nucleotide sequencing (Genetic Analyzer 3130XL, Applied Biosystems). The resultant sequences were aligned (Bioedit Software 7.0.0) and BLAST against databases for the specific identification of the ectoparasites and the endosymbionts respectively. The phylogenetic tree was constructed by maximum likelihood method with 1,000 bootstrap replicates using Mega Version X. Intra- and Inter-species analysis of genetic distance values based on the 16S rRNAgene sequences between CLEsdetected in R. sanguineus, R. haemaphysaloides and H. bispinosa ticks and other strains of CLEsdocumented in GenBank was done using Mega Version X.
RESULTS
Morphological characteristics of the ectoparasites and molecular taxonomy of the ticks
The map of the study sites are given in Fig. 1. Out of the 304 animals screened (cows = 183, dogs = 96, goats = 17, cats = 3, rabbits = 2, horse = 3) a total of 261 (85.9%) animals were observed to have ectoparasite infestation. Of the 954 ticks collected, 275 were males, 580 were females, 65 were nymphs and 34 were larvae. The 5 tick species identified in our study are as follows, R. sanguineus, R. annulatus, R. haemaphysaloides, Haemaphysalis intermedia, and H. bispinosa. The particulars of the hosts, ectoparasites collected and ectoparasite indices are given in Table 1. By molecular taxonomy, no discordance with the morphological identification of the tick species was observed.
Table 1.
Details on the hosts and ectoparasites retrieved in each study site
Details of the endosymbionts detected in the ticks, eggs and larva
A total of 53 pools of field-collected ticks, 2 pools of eggs and 6 pools of lab-reared larvae were screened for the presence of endosymbionts such as Wolbachia, Coxiella-like, Francisella-like, and Midichloria mitochondrii. All the pools were tested negative for Wolbachia, Francisella-like, and Midichloria mitochondrii endosymbionts. Among the field-collected ticks, eight pools were tested positive for CLEs which includes two pools of R. haemaphysaloides, three pools of R. sanguineus from dogs, and one pool of R. sanguineus tickscollected from a goat. Interestingly, CLEs positivity was also observed in two pools of H. bispinosa ticks collected from cattle. Both the male and female ticks were tested to harbor CLEs in all the species of the ticks.
BLAST analysis revealed a similarity of 99.78-100% and 98.51% with the database sequences of CLEs reported in R. sanguineus and Haemaphysalis sp. of ticks, respectively. According to Brenner et al., (2021), CLEs infesting soft ticks are categorised under Clade A and those infesting hard ticks are categorised under Clades B and C in phylogenetic analysis (12). In view of the absence of soft tick infestation in the animals screened, Clade A sequences were excluded for phlyogenetic analysis. The samples are named starting with an identification number followed by species name abbreviated as RS, RH and HB representing R. sanguineus, R. haemaphysaloides and H. bispinosa ticks respectively. This is followed by the gender of the tick represented as either M or F for male and female respectively and by the region from where the ticks were collected. The eggs of sample 7.2 were reared under laboratory conditions for testing the transovarial transmission and are represented as TOT eggs1, eggs2, larva and tick. As expected, the CLEs detected in our study belonged to Clade B / CLEs of Haemaphysalis sp. and Clade C/ CLEs of Rhipicephalus sp. ticks, respectively (Fig. 2). Construction of the Phylogenetic tree based on the country of the report revealed that the CLEs identified in this study were highly similar to those lineages reported from Asian countries (Fig. 3). Analysis of intra- and inter-species genetic distances revealed that the CLEs detected from R. sanguineus and R. haemaphysaloides were almost similar with a divergence accounting for 0.4% (Table 2).However, considerable genetic divergence (2.7-3.16%) was observed between the CLEs detected in R. haemaphysaloides, R. sanguineus, and H. bispinosa ticks,respectively.
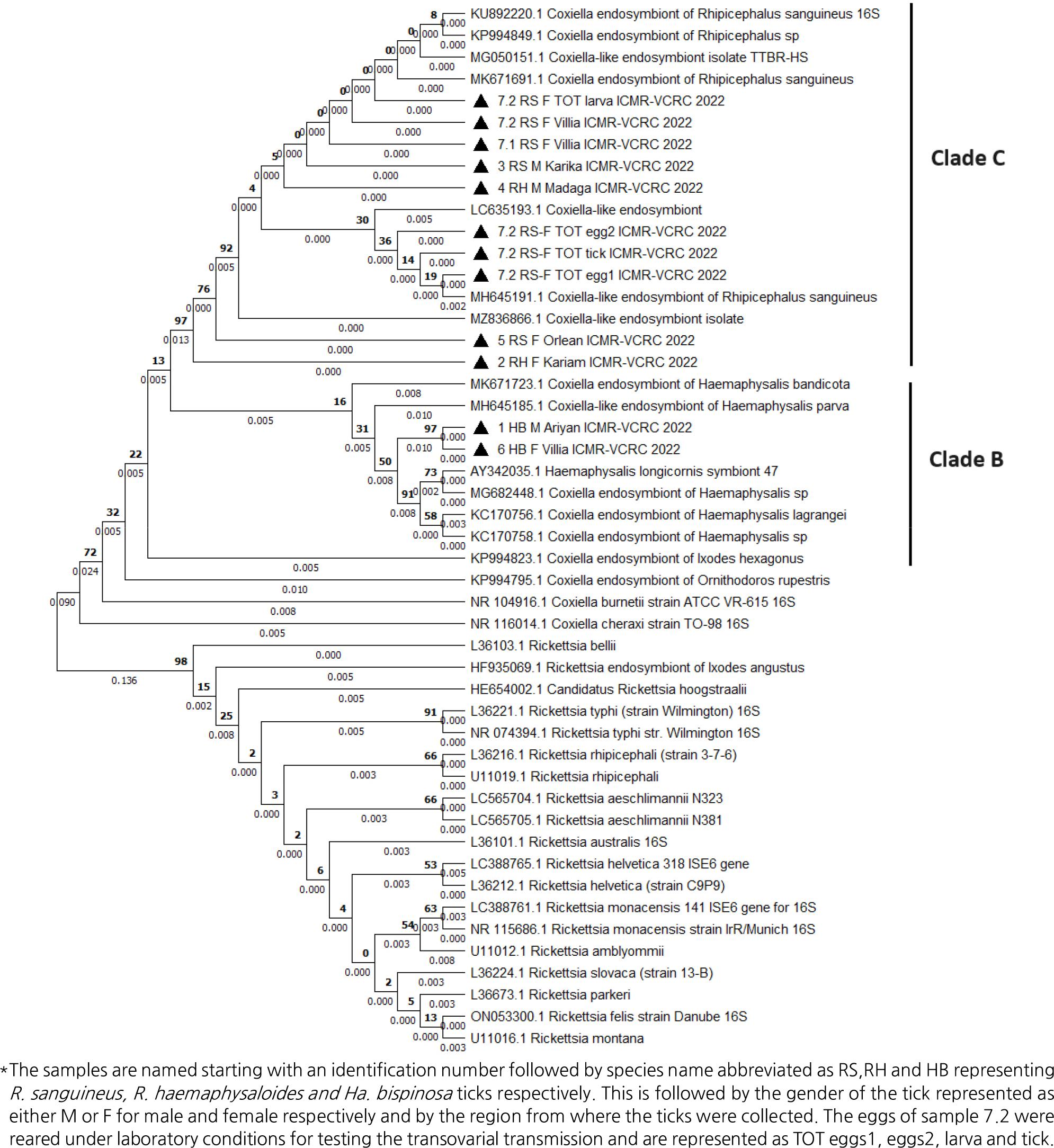
Fig. 2
Phylogenetic distribution of Coxiella-like endosymbionts based on 16S rRNA nucleotide sequences. Phylogenetic tree was constructed with 1000 bootstrap replicates by Maximum likelihood method using MEGA X. Coxiella-like endosymbionts detected in this study are represented by solid triangle in front of the label.

Fig. 3
Phylogenetic distribution of Coxiella-like endosymbionts using 16S rRNA nucleotide sequences based on geographical location. Phylogenetic tree was constructed with 1000 bootstrap replicates by Maximum likelihood method using MEGA X. Coxiella-like endosymbionts detected in this study are represented by solid triangle in front of the label.
Table 2.
Intra- and inter-species analysis of genetic distance values based on the 16S gene sequences between the Coxiella-like endosymbionts detected in R. sanguineus, R. haemaphysaloides and H. bispinosa ticks and with the other strains of Coxiella-like endosymbionts from GenBank
*The samples are named starting with an identification number followed by species name abbreviated as RS,RH and HB representing R. sanguineus, R. haemaphysaloides and Ha . bispinosa ticks respectively. This is followed by the gender of the tick represented as either M or F for male and female respectively and by the region from where the ticks were collected. The eggs of sample 7.2 were reared under laboratory conditions for testing the transovarial transmission and are represented as TOT eggs1, eggs2, larva and tick.
Vertical transmission of CLEs in R.sanguineus ticks
The laboratory-reared ticks, eggs, and larvae derived from R. sanguineus were also tested positive for the presence of CLEs. By phylogeny, the CLEs detected in parental ticks, eggs, and larvae derived from R.sanguineus clustered under the clade C, the CLEs of Rhipicephalus sp. of ticks.
DISCUSSION
Endosymbionts such as Wolbachia, Coxiella-like, Francisella-like, and Midichloria mitochondrii were reported in hard and soft ticks (9, 10, 11). In our study, among the 4 endosymbionts screened, the presence of CLEs was observed in R. sanguineus, R. haemaphysaloides and H. bispinosa ticks. Our finding of CLEs in R. sanguineus is similar to the reports of Lalzer et al.(2012) (13) and Duron et al.(2018) (5). In addition, such reports of CLEs in R. sanguineus and R. microplus have been reported from Northeast India (14) and Uttarakhand, North India (15).
BLAST analysis revealed that the CLEs detected in R. sanguineus had 99.78-100% similarity with the sequences reported in R. sanguineus from Thailand (16), and Australia (17). Phylogenetic analysis of the CLEs detected in R. sanguineus and R. haemaphysaloides clustered with the clade C of CLEs reported in India, Japan, China and Israel (12). We first report the presence of an endosymbiont in H. bispinosa ticks. The CLEs detected in H. bispinosa was observed to have 98.51% sequence similarity with the endosymbionts reported in Haemaphysalis ticks from Asian countries and clustered with the clade B of endosymbionts (12).
A trend towards genetic similarity in CLEs based on tick hosts was observed. However, Intra- and inter-species genetic distance analysis revealed that CLEsdetected in R. sanguineus, R. haemaphysaloides, and H. bispinosa ticks have wider genetic differences (Table 1). In our study, the ticks that tested positive for CLEs were collected from 3 different vertebrate hosts such as cattle, goats, and dogs. The major limitation of our study is that we have not tested the animals for CLEs, as there are reports of the presence of endosymbionts in animals (14, 18). The role of vertebrate hosts in the circulation of endosymbionts in the tick hosts is yet to be elucidated.
Rialch et al. (2022) (15), demonstrated the transovarial transmission of endosymbionts in Rhipicephalus microplus ticks. We have also demonstrated the vertical transmission of CLEs in eggs and larvae derived from field-collected and laboratory-maintained R. sanguineus ticks. The genetic similarity of CLEs detected from the adult females, eggs, and larvae further confirms the transovarial transmission of CLEs in R. sanguineus. Though there are reports on the high prevalence of CLEs in female ticks, in our study we observed that both the male and female ticks harbored CLEs, which might be due to the vertical transmission of CLEs (2, 15).
In this study, we did not observe the presence of the endosymbiont, Wolbachia in any of the ectoparasites analysed. It was reported that Wolbachia gains entry into the ticks through incidental infection of ticks by a parasitic wasp Ixodiphagus hookeri, which is responsible for detection of Wolbachia in ticks (19). In addition, the lack of Francisella-like endosymbionts in our study is in line with the reports of Rialch et al (2022) (15). Gerhart et al. (2016) (20)reported that Francisella-like endosymbionts could be detected in ticks infesting rodents. Midichloria mitochondrii, reported in Ixodes, Rhipicephalus, Hyalomma, and Amblyomma ticks (4) was not found in Rhipicephalus and Haemaphysalis ticks in our study.
A longitudinal study would give a better insight into the influence of season on the ectoparasite and its endosymbiont diversity. A knowledge gathered by encompassing all the factors such as season, animal hosts, tick hosts, and endosymbionts, might help to develop newer strategies targeting the CLEs to mitigate the tick infestation in animals.





