INTRODUCTION
Scrub typhus is a mite-borne disease caused by infection with Orientia tsutsugamushi, transmitted to animal hosts by the larval stage of Leptotrombidium mites (1, 2). Clinical symptoms vary from mild to severe, commonly including fever, headache, rash, nausea, lymphadenopathy, and eschar formation at the site of the mite bite (3). Delayed antibiotic treatment with tetracycline, chloramphenicol, or macrolides often results in severe complications, such as pneumonia, meningitis, multi-organ failure, and acute respiratory distress syndrome. In the pre-antibiotic era, the mortality rate reached up to 40–50% (4, 5, 6).
Scrub typhus is primarily endemic within the “tsutsugamushi triangle”, encompassing Southeast Asia, East Asia, South Asia, and the Western Pacific. It is estimated that over a million cases occur annually, with nearly one billion people at risk in these regions (7). Recent reports indicate scrub typhus cases are also emerging in other continents beyond traditional endemic areas, including South America and Africa (8, 9, 10, 11, 12).
In South Korea, the first reported scrub typhus cases occurred during the Korean War in 1951, with a re-emergence since 1986 (13). The incidence rate has steadily increased, and scrub typhus was classified as a notifiable infectious disease in 1994. Between 2001 and 2023, a total of 143,035 cases were reported to the Korea Centers for Disease Control and Prevention (KCDC). Cases are primarily reported from rural areas with mountainous and agricultural landscapes; however, recent studies also indicate a gradual rise in urban cases, such as in Seoul metropolitan city (14). Understanding the factors driving these changes in endemic patterns is crucial from a public health perspective, as it facilitates early preventive measures and the development of effective response strategies.
Several environmental factors are known to influence scrub typhus epidemiology, including climatic, ecological, and social factors (15, 16, 17, 18, 19, 20, 21, 22). These factors primarily affect the life cycle and population density of chigger mites and the risk of human exposure (23). Favorable climatic conditions can extend mite activity periods and accelerate their reproductive cycles, leading to increased populations (24, 25). For instance, a study found that each 1°C increase in average monthly temperature correlated with a 3.8% rise in scrub typhus incidence (26). Humidity and precipitation also play important roles, as high relative humidity supports mite development by increasing their lifespan and population (27). A study in Chile demonstrated that chigger survival and reproduction rates were optimal at relative humidity above 50%, while rates declined when humidity dropped below 50% (28). The local density of chigger mites, which thrive in moist, organic-rich environments such as farmland and forests with leaf litter, is also closely associated with scrub typhus prevalence (23).
In South Korea, high incidence rates are strongly associated with agricultural activities, particularly during the autumn harvest season when mite activity peaks, increasing infection risks among farmers (29). Social factors, including population aging and increased outdoor activities, are also linked to the rising incidence of scrub typhus. The aging rural population, which largely engages in farming, represents a growing risk group due to weakened immune systems upon aging (7). Additionally, the increase in outdoor recreational activities, even among urban populations, contributes to rising scrub typhus cases by increasing exposure to mite habitats in mountains and grassy fields during the endemic season. Given these various environmental and social factors, which have changed significantly in recent years, scrub typhus has seen notable shifts in its endemic pattern in South Korea (30, 31).
To investigate and characterize these recent changes in scrub typhus epidemiology in South Korea, we analyzed case data alongside environmental factors, including climatic and ecological variables, from 2001 to 2023. This approach allows us to assess regional and seasonal variations and recent incidence trends, which could provide valuable insights for reducing the potential risks associated with scrub typhus.
MATERIALS AND METHODS
To analyze the changing epidemiological trends of scrub typhus in South Korea, we examined the relationship between the incidence rate of scrub typhus and various environmental and demographic factors. The incidence data of scrub typhus were obtained from the Korea Centers for Disease Control and Prevention (KCDC) open database (https://dportal.kdca.go.kr) and climate data were collected from the Korea Meteorological Administration open portal (https://data.kma.go.kr).
A linear correlation analysis was conducted to assess the association between the incidence rate of scrub typhus and each of these factors. Monthly average temperature was used to investigate its influence on the changing trends in scrub typhus incidence, while demographic variables such as sex and age group were also analyzed to determine population-level vulnerabilities. Pearson’s correlation coefficient was calculated to measure the strength and direction of the linear relationship among the variables. Statistical analyses were performed using GraphPad Prism version 8.0.2, and results were considered statistically significant at a p-value of less than 0.05 in linear regression analysis.
RESULT
From 2001 to 2023, a total of 143,035 cases of scrub typhus were reported in South Korea. The annual number of cases shows a trend of gradual increase, with intermittent stepwise changes approximately every 6-7 years (Fig. 1A). Between 2005 and 2011, an average of 5,879 cases occurred annually, which rose to an average of 9,708 cases between 2012 and 2017, indicating an approximately 61% increase in annual cases between these two periods. Overall, there was an average annual increase of 161 cases throughout the study period, and the rise in incidence rate was statistically significant (p=0.0453) (Fig. 1A). Case fatality also showed a significant upward trend (0.013% per year) from 2011 to 2023 (Fig. 1B), reaching its highest levels in 2022 and 2023, where it exceeded 0.3% of case fatality.
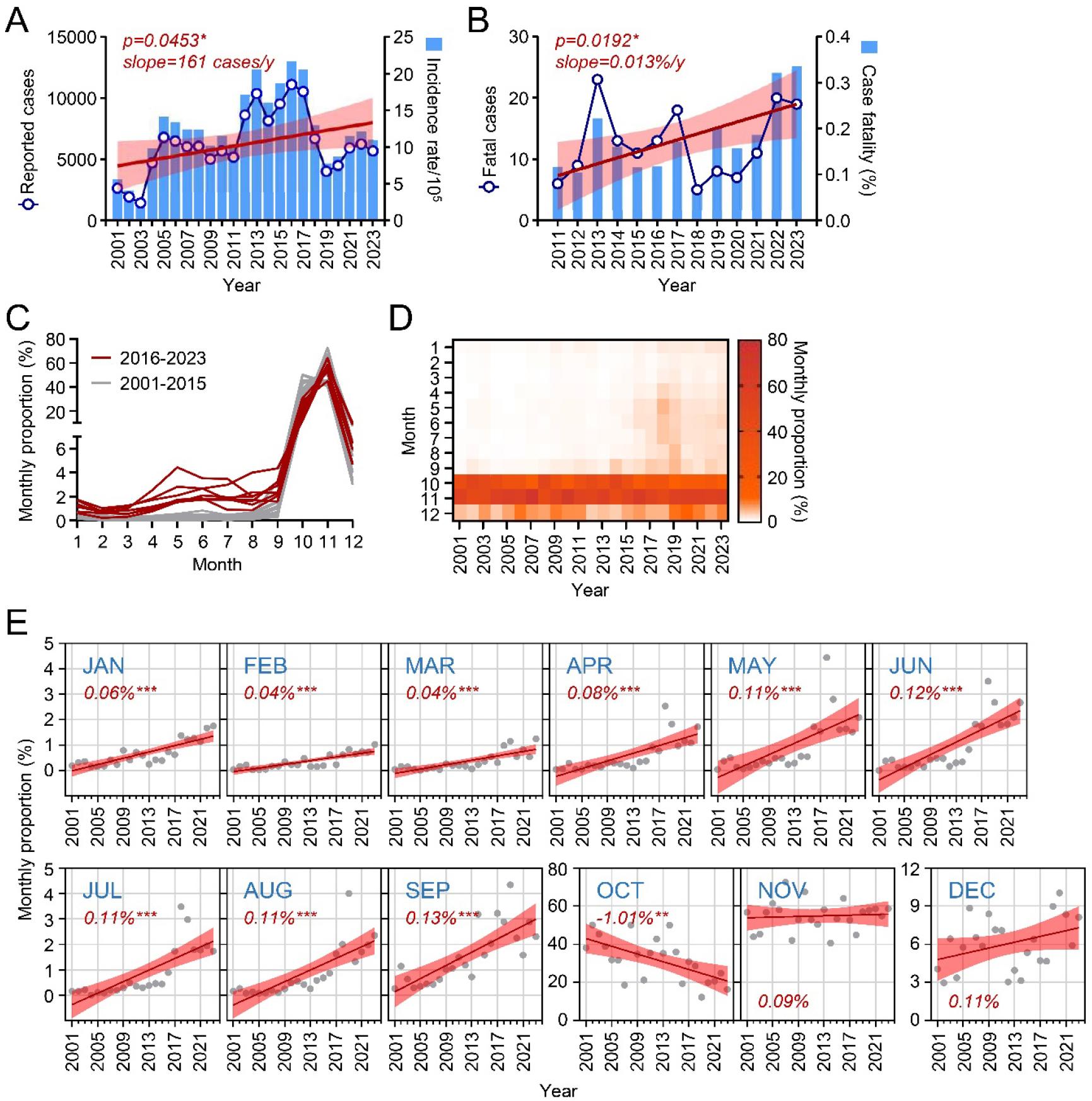
Fig. 1
Epidemiological trends of scrub typhus cases in South Korea, 2001–2023. (A) Reported cases, incidence rate, and linear regression of the incidence rate (red line with shaded 95% confidence intervals). (B) Reported fatal cases, case fatality rate, and linear regression of the fatality rate (red line with shaded 95% confidence intervals). (C) Annual trends in the monthly proportion of scrub typhus cases, shown as two periods: 2001–2015 (grey lines) and 2016–2023 (red lines). (D) Heatmap showing monthly proportions of scrub typhus cases across the survey years. (E) Trends in monthly proportions over the study period, represented by linear regression (red line) with shaded 95% confidence intervals.
To investigate seasonal prevalence, we examined the monthly distribution of reported cases each year (Fig. 1C and 1D). Traditionally, scrub typhus cases peak in October and November, accounting for 40-60% of total cases. However, the proportion of cases occurring from January to September significantly increased from 2001 to 2023, while October showed a significant decrease, and November remained unchanged (Fig. 1E). These findings suggest a shift in scrub typhus seasonality, with cases appearing earlier in the year.
We further analyzed the demographic distribution of scrub typhus cases by sex and age. From 2001 to 2023, there were 54,831 male and 89,225 female cases, indicating an incidence rate 1.6 times higher in females than males (p=0.0006, Fig. 2A). Incidence rates increased sharply with age, particularly among those over 50 (Fig. 2B). Both males and females showed increasing incidence trends over the years (Fig. 2C). The average case fatality rate was 0.17%, with no significant difference between males and females (Fig. 2D). Fatalities were primarily reported among those over 50, with mortality rates rising with age (Fig. 2E). Notably, a general increase in fatality rates over time was observed, especially among females (p=0.0214) (Fig. 2F), identifying elderly females as the primary risk group with elevated mortality over the past two decades.
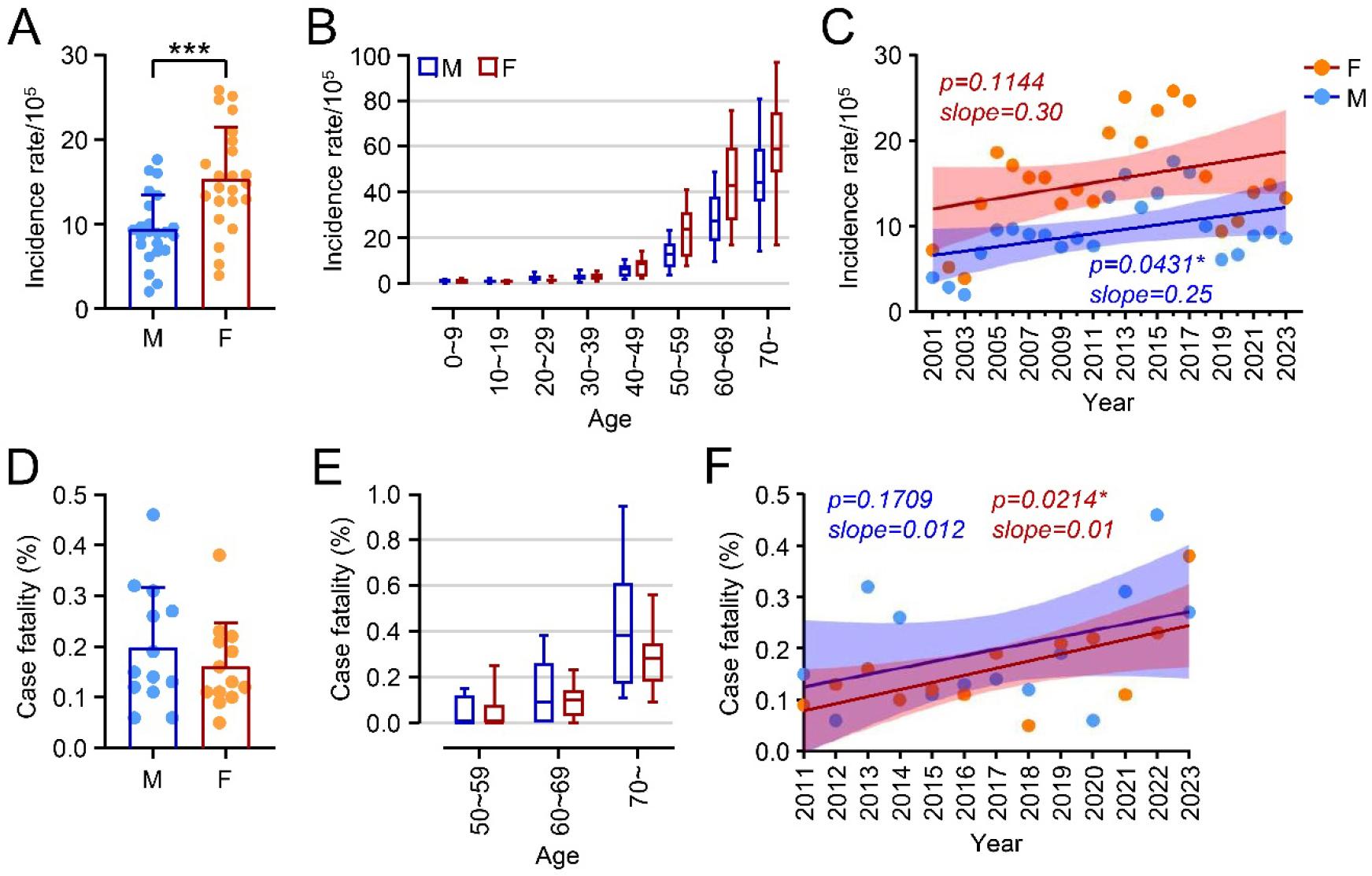
Fig. 2
Sex and age associations of scrub typhus cases in South Korea, 2001–2023. (A) Incidence rate of total scrub typhus cases by sex across the survey period, with each dot representing the annual incidence rate. M: male, F: female. (B) Incidence rate of scrub typhus cases by age group. (C) Trends in incidence rates over the study period, separated by sex. Case fatality rate by sex (D) and age group (E). (F) Trends in case fatality rates over the study period, separated by sex. ***: p<0.001.
To explore regional prevalence, we examined the accumulated scrub typhus cases across South Korea’s nine provinces from 2001 to 2023. The highest accumulated incidence rate was recorded in Jeollabuk-do (JB, averaging 939.8 cases per 100,000), and the lowest in Gyeonggi-do (GG, averaging 66.2 cases per 100,000) (Fig. 3A and 3B). Although regional differences in incidence were not statistically significant, scrub typhus tended to be more prevalent in southern areas. Differences in case fatality rates among provinces were also not significant, although Gyeongsangbuk-do (GB) exhibited a relatively higher rate (0.83%) than other provinces (Fig. 3C). Analyzing regional trends over time, only three southern provinces—Jeollanam-do (JN, p=0.0032), Gyeongsangnam-do (GN, p=0.0083), and Jeju-do (JJ, p=0.0003)—showed significant increases in incidence, suggesting that climate factors might be associated with these rising trends.
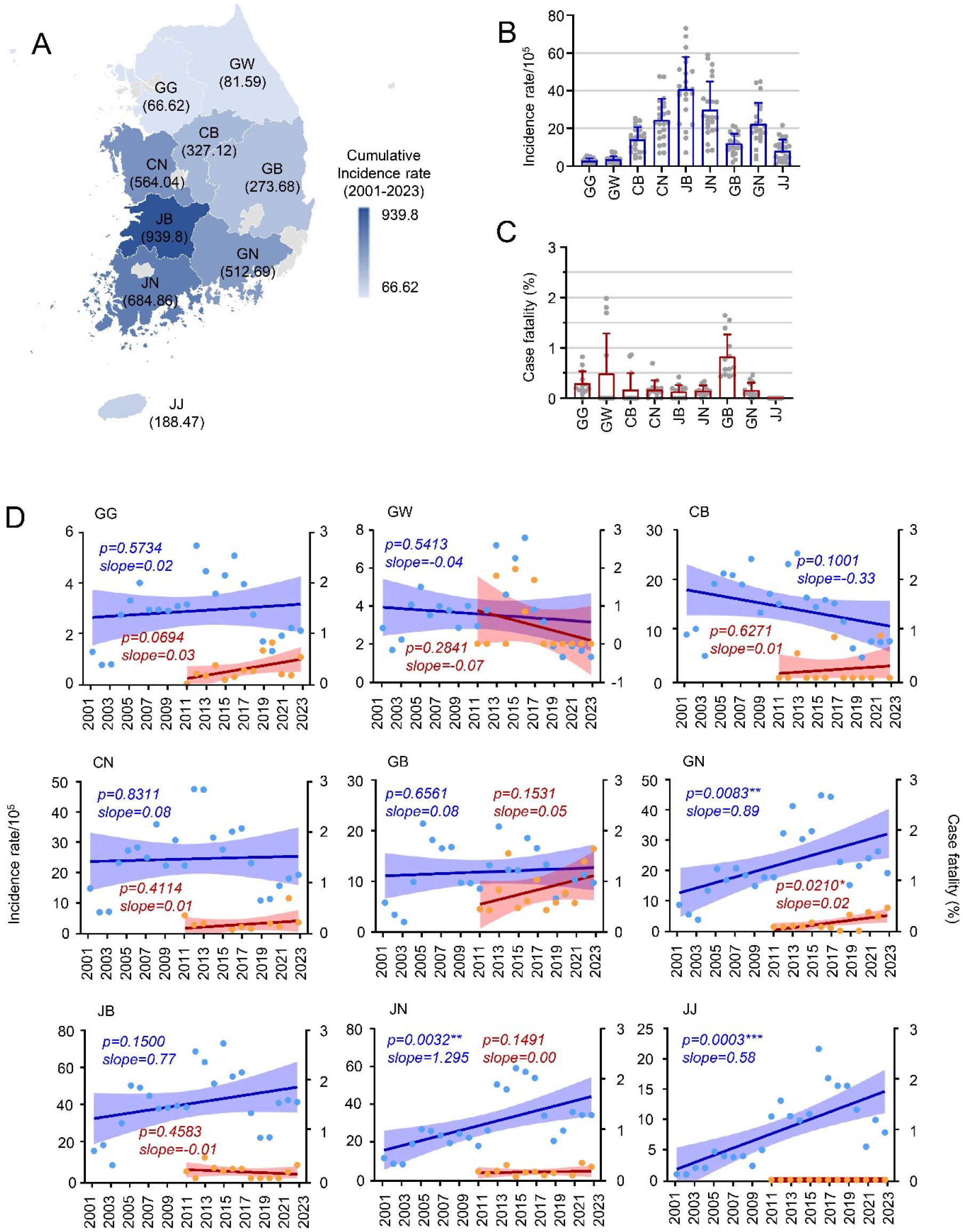
Fig. 3
Regional trends in incidence rate and case fatality of scrub typhus in South Korea, 2001–2023. (A and B) Cumulative incidence rate of scrub typhus across the indicated provinces. (C) Case fatality rates by province. (D) Trends in incidence rate (blue) and case fatality (red) over time in the indicated provinces. GG: Gyeonggi-do, Seoul, and Incheon; GW: Gangwon-do; CB: Chungcheongbuk-do; CN: Chungcheongnam-do and Daejeon; GB: Gyeongsangbuk-do and Daegu; GN: Gyeongsangnam-do and Busan; JB: Jeollabuk-do; JN: Jeollanam-do and Gwangju; JJ: Jeju-do.
To understand environmental influences, we examined annual changes in mean temperature and precipitation from 2001 to 2023. The annual mean temperature significantly increased from 12.6°C to 13.9°C over this period, showing a strong positive correlation (r=0.7259p<0.0001), while annual precipitation showed no significant change (Fig. 4A). Correlation analysis revealed a moderate positive association between annual mean temperature and both incidence and fatality rates (incidence: r=0.2488; fatality: r=0.4827), though without statistical significance, suggesting a moderate impact of temperature increases on scrub typhus trends (Fig. 4B and 4C).
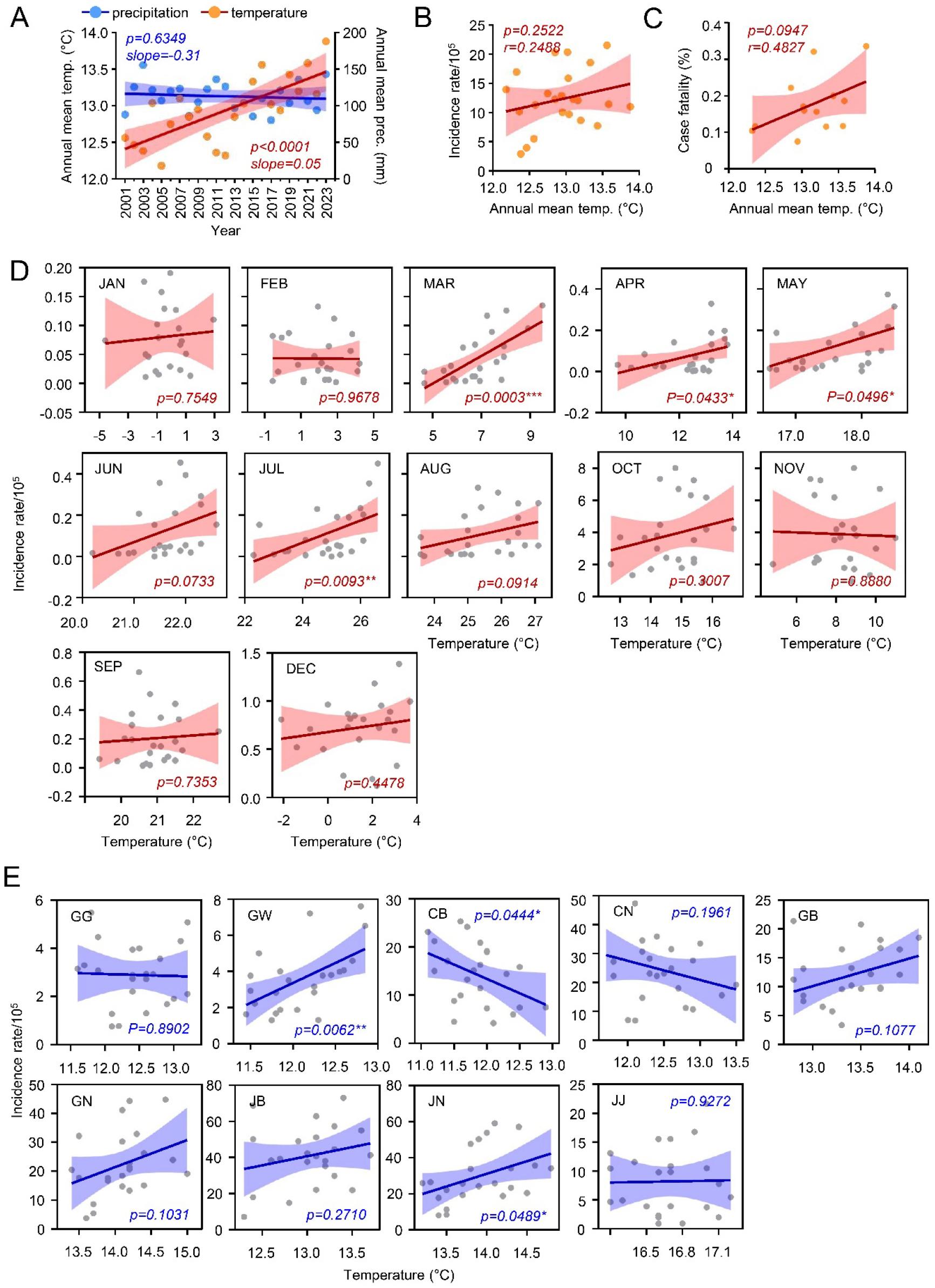
Fig. 4
Correlation between climatic conditions and scrub typhus cases in South Korea, 2001–2023. (A) Correlation between scrub typhus incidence rate and climatic conditions throughout the survey period. (B) Correlation between annual mean temperature and incidence rate of scrub typhus. (C) Correlation between annual mean temperature and case fatality rate of scrub typhus. (D) Correlation between monthly mean temperature and incidence rate over the survey period. (E) Correlation between the annual mean temperature and incidence rate of scrub typhus in the indicated provinces.
Given the regional and seasonal variations observed, we further analyzed the relationship between temperature changes and incidence rates by season. Positive correlations between monthly mean temperature and rising incidence rates were observed consistently during spring (March: r=0.6866, p=0.0003; April: r=0.4248, p=0.0433; May: r=0.4139, p=0.0496) and summer (June: r=0.3805, p=0.0733; July: r=0.5301, p=0.0093; August: r=0.3602, p=0.0914). In contrast, temperature had little correlation with incidence rates in autumn (October: r=0.2256; November: r=-0.03110) and winter (December: r=0.1665; January: r=0.0689; February: r=-0.0089) (Fig. 4D). These results suggest that temperature changes may be driving a seasonal shift in scrub typhus cases.
Another critical environmental factor that influences the incidence of scrub typhus is relative humidity. While rainfall represents the physical quantity of precipitation, relative humidity reflects the degree of water vapor saturation in the atmosphere, providing a sustained environment essential for the survival and activity of mites.
To further examine this environmental factor, we analyzed the average relative humidity from 2001 to 2023. The analysis revealed that there is a gradual increase in average relative humidity, raging from 80% to 88% (Fig. 5A, p=0.059). The correlation analysis demonstrated a significant and positive association between relative humidity and incidence rates (Fig. 4B, p= 0.012, r= 1.152). In contrast, the annual change of humidity showed a strong negative correlation with case fatality (Fig. 5C, p= 0.028, r= -0.025). Next, we analyzed these correlations across different seasons to identify potential seasonal variations.
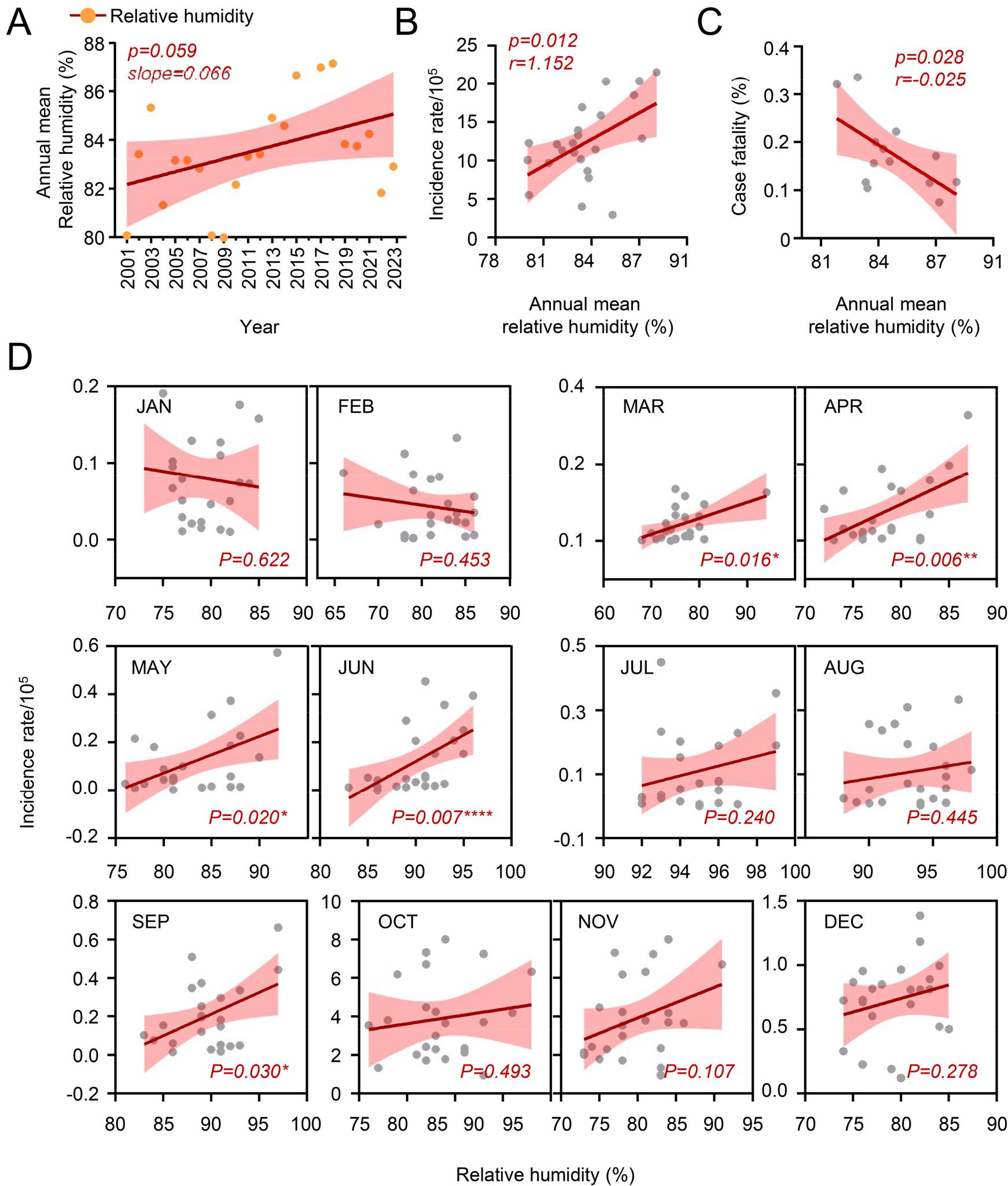
Fig. 5
Correlation between relative humidity and scrub typhus cases in South Korea, 2001–2023. (A) Correlation between scrub typhus incidence rate and relative humidity throughout the survey period. (B) Correlation between annual relative humidity and incidence rate of scrub typhus. (C) Correlation between annual relative humidity and case fatality rate of scrub typhus. (D) Correlation between monthly mean humidity and incidence rate over the survey period.
A weak negative correlation was observed in the winter month (January: r= -0.002, February: r= -0.002). and a slight positive correlation was observed between humidity and incidence rates during the other seasons (March: r=0.004, April: r=0.012, May: r=0.015, Jun: r=0.022, July: r=0.015, August: r=0.006, September: r=0.022, October: r=0.076, November: r=0.159, December: r=0.021). Notably, a significant correlation between humidity and incidence rates was identified during spring to early summer and autumn, excluding summer and winter seasons (March: p=0.016, April: p=0.006, May: p=0.020, June: p=0.007, September: p=0.030) (Fig. 5D). Based on these findings, humidity also appears to be a potential driving factor influencing the changing patterns of scrub typhus.
Finally, examining the association between annual mean temperature and regional incidence rates revealed significant positive correlations in Jeollanam-do (r=0.4510, p=0.0489) and Gangwon-do (GW, r=0.5600, p=0.0062), and a negative correlation in Chungcheongbuk-do (CB, r=-0.4229, p=0.044) (Fig. 4E). These findings suggest a complex relationship between temperature and regional prevalence of scrub typhus, potentially influenced by other environmental factors.
DISCUSSION
This study investigated epidemiological trends of scrub typhus in South Korea from 2001 to 2023 and examined the potential influence of environmental factors on the disease’s rising incidence. Key findings include a clear increasing trend in scrub typhus cases over the years, despite periodic fluctuations occurring every 6-7 years, along with a gradual rise in case fatality rates (Fig. 1). Notably, from January to September, the monthly distribution of reported cases has shown significant growth in recent years, especially from 2016 to 2023. In contrast, cases reported during the peak season have either decreased (October) or remained stable (November). Regional analysis also revealed significant increases in reported cases in southern provinces, particularly in Gyeongsangnam-do, Jeollanam-do, and Jeju-do (Fig. 3), suggesting that varying environmental conditions may impact regional disease prevalence.
The influential factors driving these epidemiological changes can be categorized into three groups: climatic, ecological, and social. Climatic and ecological factors are closely linked to the life cycle of chigger mites, which are vectors of scrub typhus (4, 23, 24, 32). Meanwhile, social factors, such as population demographics (age and sex), appear to influence incidence and fatality rates more directly. Our findings showed that females had a significantly higher incidence rate than males (Fig. 2), consistent with previous studies (33). Cases increased over the years, with age contributing to incidence rates across both sexes. Among individuals over 50, females exhibited higher incidence rates than males. Although the mortality rate increased with age, no overall sex differences in mortality were observed. These results highlight that both age and sex are significant determinants of scrub typhus incidence and mortality in South Korea. Unlike trends observed in Japan, Thailand, and China, the higher incidence among women is a distinctive feature in South Korea (33). This pattern may be linked to traditional labor roles, particularly in agriculture (15). In South Korea, elderly women, especially in rural areas, engage frequently in fieldwork, often crouching or sitting, which increases exposure to mite habitats (18). Men, in contrast, typically perform agricultural tasks that involve standing or using tools, which may reduce their exposure.
Geographically, South Korea is surrounded by seas on three sides and has 70% mountainous terrain, creating diverse vegetation and environmental conditions across nine regional provinces with varying latitudes. Ecological factors strongly influence mite habitats, as forests and fields with abundant bushes and grass provide ideal conditions for mite reproduction and food sources (34, 35). Moreover, increasing wild animal or livestock density contributes to the rise in mite populations. Our study observed significant increases in scrub typhus incidence in southern provinces, particularly Gyeongsangnam-do, Jeollanam-do, and Jeju-do, over recent years. The mild climate and expansive plains in Gyeongsangnam-do and Jeollanam-do favor agricultural activities, which, along with high chigger mite populations in these areas, may facilitate disease spread. Indeed, approximately 50% of rodents tested in these areas showed scrub typhus antibodies (36). Therefore, geographical factors, particularly regions with extensive agricultural activity, likely impact scrub typhus incidence and dynamics.
Climate also plays a crucial role in the mite life cycle (23, 24). Mites thrive in high-humidity environments with mild temperatures (above 10°C) (32).
In temperate and subtropical climates, rising temperatures increase mite population density by enhancing larval survival and extending breeding periods (25).
From 2001 to 2023, the annual mean temperature in South Korea showed a significant increase, which correlated positively with incidence and fatality rates. In addition to temperature, relative humidity exhibited a substantial positive association with the rising incidence rate of scrub typhus. Notably, annual relative humidity increased sharply between 2013 and 2018 (Fig. 5A), coinciding with a sudden surge in scrub typhus incidence (Fig. 1A). This temporal shift may reflect environmental conditions that supported higher chigger mite density and/or enhanced their survival during these years. Although multiple factors contribute to these trends, climate change is likely a factor in the increased spread of vector-borne diseases at regional levels (19). For example, warmer temperatures in Colombia and Ethiopia have been associated with the upward shift of malaria distribution to higher altitudes (37). In Guangzhou, China, a 1°C temperature rise was associated with a 15% increase in scrub typhus cases (38). Similarly, a study conducted in Yunnan Province, China, found that scrub typhus cases were most prevalent when relative humidity ranged from 78% to 82%, suggesting that rising temperatures and optimal humidity conditions together create an environment to expand geographic distribution and vector activity for disease transmission (39). However, we observed substantial variability in the relationship between incidence rate and annual mean temperature across provinces, likely due to variations in population density, age demographics, agricultural practices, and regional climate changes.
Further research is needed to assess the association of climatic, ecological, and social factors with scrub typhus incidence on a regional basis. This would help identify key conditions contributing to the disease and support the development of targeted healthcare responses and future trend predictions. Integrating natural and anthropogenic factors in our approach will improve our understanding of the complex dynamics of scrub typhus and inform the establishment of more specific preventive measures.




