INTRODUCTION
Antibiotic resistance is a serious global crisis that affects public health (1). According to the recent statement of World Health Organization (WHO), the mortality rate from antimicrobial-resistant infections may reach 10 million by 2050 (2). Plazomicin (formerly ACHN-490), an aminoglycoside (AG), has demonstrated efficacy against gram-negative pathogens that exhibit a broad array of resistance mechanisms (3). Plazomicin is effective against gram-positive pathogens, such as methicillin-resistant Staphylococcus aureus (including aminoglycoside-resistant strains), and gram-negative pathogens, such as extended-spectrum beta-lactamase (ESBL), multidrug-resistant (MDR) Acinetobacter, MDR Pseudomonas, among other AG resistant pathogens (4). The increase in multidrug resistant pathogens has increased the demand for new drugs (5, 6). Novel antibiotics are required to treat infections because antibiotic resistance has a dangerous effect on community health (7). The limited availability of antibiotics for treating complicated MDR infections has created a need for a new antibacterial agents, such as plazomicin (3). Uncomplicated urinary tract infections (UTIs) are infections in the bladder and urethra which comprise the lower urinary tract. In contrast, complicated urinary tract infections (cUTIs) are UTIs occurring in the presence of a structural or functional abnormality of the urinary tract (8, 9). In the US, approximately 22% of UTIs are considered as cUTIs (8). Escherichia coli is the most common bacteria that causes cUTIs. However, Citrobacter, Enterobacter, Pseudomonas, Staphylococcus aureus, and others are isolated more often (6–20%) in cUTIs than in uncomplicated UTIs (10). AGs are broad spectrum bactericidal antibiotics used to treat nosocomial respiratory tract infections and cUTIs (3). Plazomicin was approved by the United States Food and Drug Administration (US FDA) in June 2018 for treating cUTIs, including pyelonephritis (11). The Center for Disease Control and Prevention (CDC) estimated that approximately 2.8 million antimicrobial-resistsant cases are reported in the US annually, resulting in over 35,000 deaths (12). In the CDCs’s 2019 antibiotic resistance threats report, 18 microorganisms were listed as serious and urgent threats to human health, majority of which were gram-negative microorganisms, including MDR Enterobacterales (12). Plazomicin was developed to overcome this resistance mechanism and has proven to be effective against MDR Enterobacteriaceae in vitro(13, 14). AGs are an alternative treatment option for cUTIs caused by MDR organisms. The surveillance data from US from 2014–2015 showed that approximately 80% of the carbapenem-resistant Enterobacteriaceae isolates tested positive for one or more aminoglycoside-modifying enzymes (AMEs). AGs such as gentamicin, amikacin, and tobramycin exhibited reduced activity against these isolates, with only 49.4%, 59.7%, and 0% susceptibility to gentamicin, amikacin, and tobramycin, respectively (15). Plazomicin suppresses protein synthesis in bacteria and exhibits efficient bactericidal efficacy against numerous ESBL producing and aminoglycoside- and carbapenem-resistant bacterial isolates (16). Unfortunately, there are few reviews of the literature on this topic. This review focuses on the clinical use of plazomicin in treating diseases caused by pathogens that are resistant to traditional antibiotics.
CHEMISTRY AND MODE OF ACTION
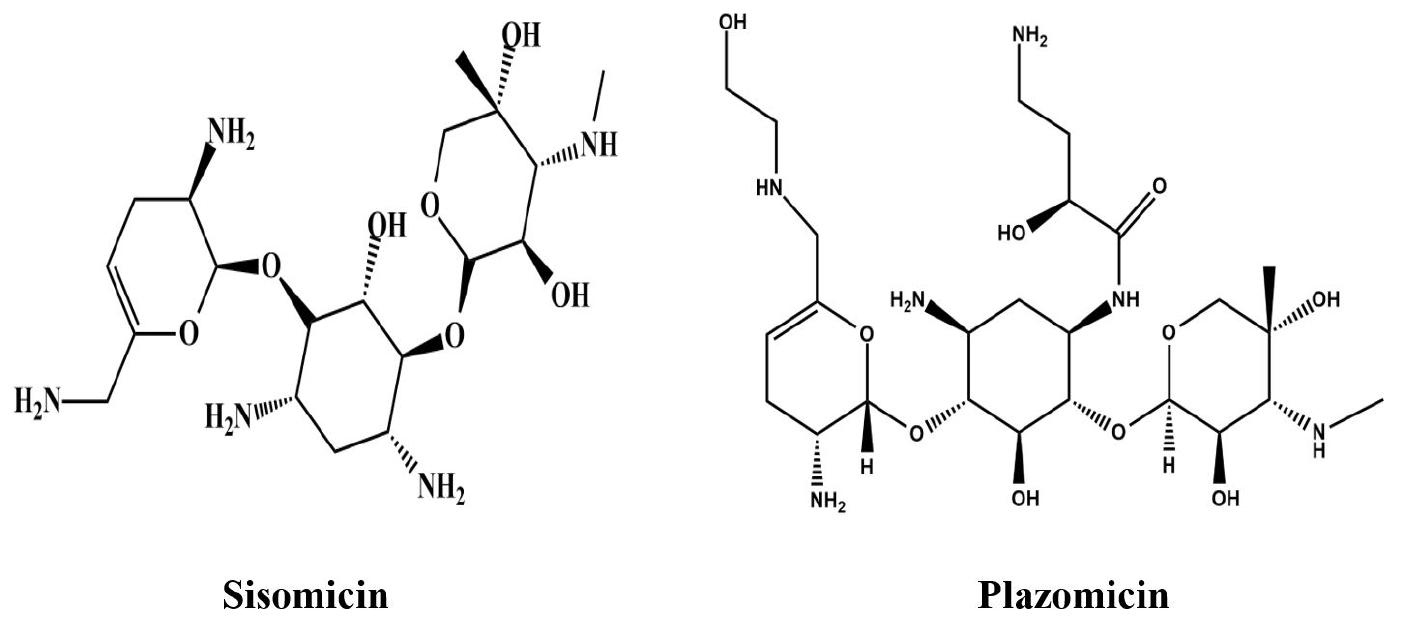
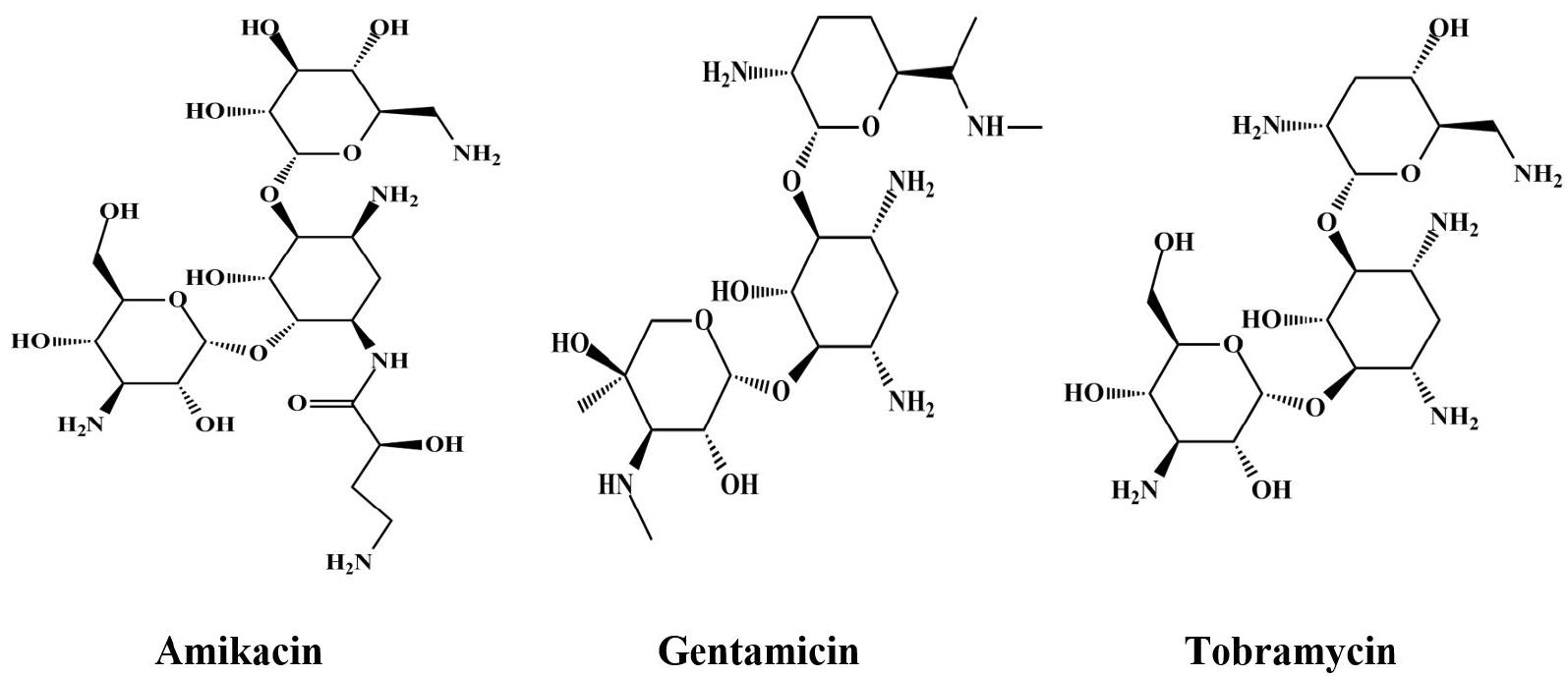
A new AG, plazomicin, is derived from sisomicin, a natural AG (Fig. 1) (17, 18). The molecular formula of plazomicin is C25H48N6O10(19). The structure of plazomicin and other AGs are shown in in Fig. 2.
The aminoacyl-tRNA recognition site (A-site) of the 16S rRNA (a part of the 30S ribosomal subunit) is the binding site for AGs. Aminoglycosides are hydrophilic and cationic molecules that inhibit ribosomal protein synthesis in a concentration- dependent manner by interrupting nascent protein sequence elongation during translation (2, 7). Reportedly, AGs enter the cells of gram-negative bacteria through porin channels; moreover, it is considered that they may enter the cells by disrupting the outer lipopolysaccharide membrane (2). Like other AGs, plazomicin demonstrates antibacterial efficacy by attaching to the 30S ribosomal subunit of bacteria, resulting in the inhibition of bacterial protein synthesis (20). Although plazomicin has structural conformity with sisomicin, it exhibits some unique structural features (17). Plazomicin is distinguished from traditional AGs by three main structural modifications that confer protection against enzymatic deactivation by various AMEs (Fig. 3) (21, 22). Lack of hydroxyl group in position 3′ as well as 4′ shields plazomicin from two AMEs, one of them is O-nucleotidyltransferase ANT (4′) that affects tobramycin and amikacin. The other is O-phosphotransferase APH (3′), which only affects amikacin (21, (23). The incorporation of an unsaturated hydroxyethyl moiety at position 6 gives a protection against N-acetyltransferase AAC (6′) which is liable for resistance to gentamicin, amikacin, and tobramycin (21, 22, 23). Its structure contains a substitution at the N-1 position along with 4-amino-2-hydroxybutanoic acid. This substitution blocks ANT (2′′) and AAC (3) rendering them resistant to both tobramycin and gentamicin (23). The AAC (2′)-I (expressed chromosomally in some Providencia stuartii organisms) is the only identified AME among gram-negative organisms that exhibits activity against plazomicin (11). The structural differences in plazomicin can protect it from all types of clinically relevant AMEs but not from 16S rRNA methyltransferases (11). Sometimes, these AMEs are observed in carbapenem- resistant and ESBL-producing Enterobacteriaceae, and the stability of plazomicin against these enzymes results in more enhanced susceptibility rates compared to other AGs (21).
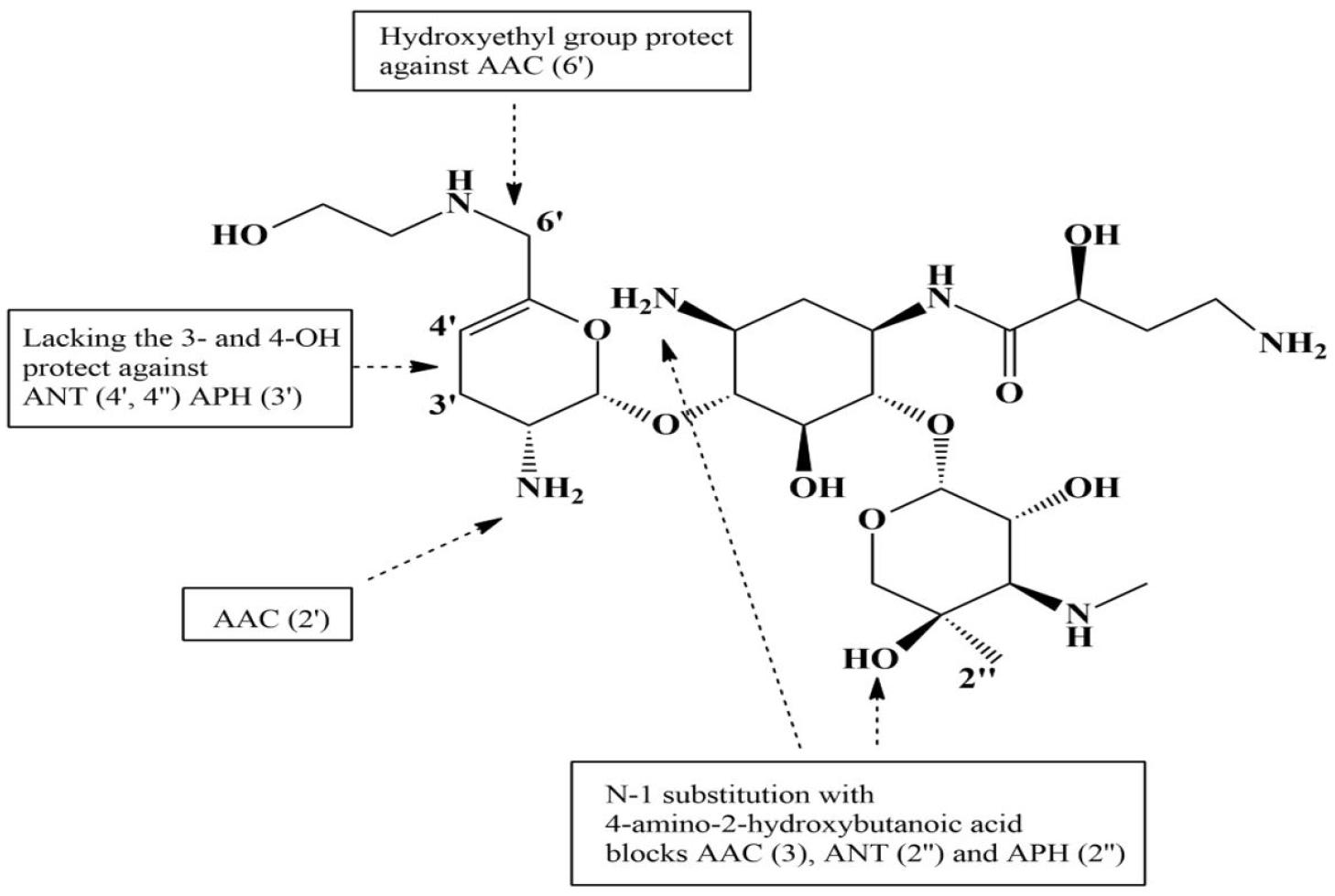
Fig. 3.
Structure of plazomicin and targeting of aminoglycoside modifying enzymes (AMEs) to the functional group of plazomicin. AMEs responsible for resistance to aminoglycoside are shown. They primarily target the NH2 and OH groups. The unique structural features of plazomicin that protect these amino and hydroxyl group at different positions are shown with arrows. AAC, N-acetyltransferase; ANT, O-nucleotidyltransferase; APH, O-phosphotransferase. The numbers in parentheses after AME abbreviations indicate their position; for example, AAC (6′), Aminoglycoside 6′-N-acetyltransferase.
The primary step in the penetration of AGs into the periplasm involves electrostatic attachment between the negatively charged cell membranes and positively charged AGs (19, 21, 24). Aerobic bacteria contain an oxygen-nitrogen-dependent electron transport chain that facilitates the transport of these molecules into the cell (19). The membrane-bound electron transport system is absent in obligate anaerobes, resulting in bacterial resistance (19). By targeting the aminoacyl-tRNA acceptance site of the 16S rRNA (ribosomal RNA), AGs suppress protein synthesis by interacting with three unpaired adenine residues in the decoding loop, eradicating the non-complementary adenine residues and locking them into a ‘flipped-out’ orientation similar to that seen during mRNA (messenger RNA) decoding (25, 26). The proofreading capabilities of ribosomes are reduced by these interactions, resulting in an increase in mistranslation and the induction of immature termination of peptide synthesis, which causes cell death (27, (28). As an AG derivative, plazomicin suppresses bacterial protein synthesis and shows dose dependent bactericidal action (27). In addition, the in vitro synergistic action of plazomicin has been reported when administered in combination with doripenem, cefepime, piperacillin-tazobactam, or imipenem against P. aeruginosa, and when combined with ceftobiprole or daptomycin against hetero-resistant vancomycin-intermediate S. aureus (hVISA), vancomycin-resistant S. aureus (VRSA), and methicillin-resistant S. aureus (MRSA) (27, 29).
MICROBIOLOGY
Commonly prescribed AGs (tobramycin, gentamicin, and amikacin) against gram-positive and -negative pathogens, including various types of AME and β-lactamase producing organisms, are shown in Tables 1 and 2. The comparative minimum inhibitory concentration (MIC) values of plazomicin and other AGs against gram-negative bacteria (Francisella tularensis and Yersinia pestis, among others), common gram-negative aerobes and some anaerobes (Prevotella spp. and Bacteroides only) are presented in Table 1(30, 31, 32, 33, 34, 35, (36). Table 2(37) shows the effects of selected AMEs on the MIC of plazomicin and other traditional AGs. The susceptibility break point is ≤ 2 mg/L (FDA breakpoint) for plazomicin (38). The equivalent activity of plazomicin was demonstrated against Klebsiella pneumonia, non-ESBL producing E. coli, and broad spectrum ESBL producing E. coli with 90% of isolates suppressed by an MIC value of ≤ 1 mg/L (38). Other AGs, such as tobramycin, gentamicin, and amikacin were more effective against Pseudomonas aeruginosa and Acinetobacter baumannii compared to plazomicin. Plazomicin is also less effective against these bacteria when its efficacy is compared against Enterobacteriaceae (38). Plazomicin against carbapenem-resistant Enterobacteriaceae (CRE) exhibited MIC90 and MIC50 values of 1 and 0.5 mg/L in the U.S, respectively (39). In contrast, it showed MIC90 and MIC50 values of 128 and 0.25 mg/L, in European and surrounding countries, respectively (39). Plazomicin against carbapenem susceptible A. baumannii (n = 8) and carbapenem resistant A. baumannii (n = 69) showed MIC values ranging from 0.5–16 μg/mL and 0.5–64 μg/mL, respectively (40). The AG inactivation by beta-lactam antibiotics has been observed in vivo and in vitro(41). Similar to other AGs, plazomicin showed lower in vitro efficacy against Stenotrophomonas maltophilia. It was evaluated in European countries against 99 isolates of Acinetobacter spp. The evaluation showed an MIC90 value of >128 mg/L with a susceptibility of 40.0%. Plazomicin demonstrated higher efficacy against Enterobacteriaceae than tobramycin or gentamicin. Although plazomicin is effective against these AMEs, which are the most described resistance mechanisms against AGs, it is not effective against less common 16S rRNA methyltransferases. However, plazomicin shows activity against Enterobacteriaceae which exhibits resistance mechanisms to other classes of antibiotics including metallo-beta-lactamases (42). In an evaluation of plazomicin against ESBL-producing K. pneumonia and E. coli bacteria, colistin resistant Enterobacteriaceae, and CRE, it was more effective than other AGs and was comparable to ceftazidime/avibactam and vaborbactam/meropenem (28, 31, 43, 44, 45). Plazomicin exhibits activity against gram-positive bacteria similar to that against gram-negative bacteria and works in a manner similar to that of other AGs (31). The MIC90 and MIC50 range of plazomicin were 0.5–1 µg/mL and 0.25–0.5 µg/mL, respectively, against Klebsiella, Escherichia, Enterobacter, Citrobacter, and Serratia species (11). Plazomicin was more effective against ESBL-producing Enterobacterales than were other AGs (11). The MIC90 of plazomicin was 1 mg/L against both methicillin susceptible S. aureus and MRSA. It was also effective against S. epidermidis with a MIC90 value of 0.5 mg/L (38). For plazomicin, the median MIC values of triplicate tests for different strains of E. coli were 0.5, 1, 2, 2, and 4 mg/L for the AECO 1174, AECO 1175, AECO 1177, SMH 64979, and SMH 64982 strains, respectively (46). The median MIC values of plazomicin for the different K. pneumoniae strains were 0.5, 0.5, 1, 1, and 2 mg/L for the SMH 41965, SMH 41966, AKPN 1169, AKPN 1170, and AKPN 1171 strains, respectively (46). The activity of this drug is confined to Stenotrophomonas maltophilia, A. baumannii, and P. aeruginosa with majority of in vitro data exhibiting MIC50 of ≥ 4 mg/L with upper MIC allocations of plazomicin when compared to other AGs (19).
Table 1.
Comparative MIC90 and MIC50 values of plazomicin and other aminoglycosides against gram-negative pathogens
Table 2.
Effect of selective aminoglycoside-modifying enzymes (AMEs) on MICs of plazomicin and other aminoglycosides
PHARMACOKINETICS AND PHARMACODYNAMICS
Similar to other AGs, plazomicin is weakly absorbed and requires parenteral administration. Aminoglycosides are unable to penetrate majority of cells and their volume of distribution (Vd) is approximately 25% of the total body weight, which is similar to the extracellular fluid volume (38). The mean Vd of this drug in healthy adults and patients with cUTIs was 18 and 31 L, respectively (47). In patients with cUTIs, the area under the curve (AUC) and a mean maximum serum concentration (Cmax) were 226 mg/L and 51 mg/L, respectively, gradually following a single intravenous (IV) dose (15 mg/kg) of this drug (48). The plasma protein binding of this drug is approximately 20% (38) and its primary excretion pathway is the kidney. The extent of plazomicin metabolism was not appreciable and cytochrome P450 (CYP-450) drug-metabolizing enzymes were not inhibited or induced. The mean serum elimination half-life (T1/2) of this drug is approximately 3.5 h in patients with normal renal function (49). The renal clearance of plazomicin is similar to its total body clearance. Approximately 90% of the drug was recovered in the urine in unchanged forms within a week following a 15 mg/kg single IV dose. In patients with moderate renal impairment and with creatinine clearance (CrCl) < 60 mL/min, the dose was reduced to 10 mg/kg every 24 h. In patients with acute renal impairment and CrCl < 30 mL/min, the dose was reduced to 10 mg/kg every 48 h to reduce toxicity (50). Physicians should use the equation: adjusted body weight = IBW + 0.4 × [TBW – IBW] for patients with 25% of total body weight (TBW) or more than 25% of ideal body weight (IBW) (51). The pharmacodynamic (PD) model of plazomicin for target acquisition showed that the proportion of the 24 h AUC to MIC (AUC 24: MIC) ratio was the pharmacodynamic parameter best correlated with drug efficacy (51). Plazomicin when administered at a dose of 15 mg/kg in a healthy volunteer, exhibited AUC0-24, Cmax, Vd, and T1/2 values of 263 ± 65.9 mg/L, 76.0 ± 19.6 mg/L, 18.5 ± 4.7 L, and 3.5 ± 0.5 h, respectively (46).
ADVERSE EFFECTS AND DRUG INTERACTIONS
Patients treated with plazomicin in the largest in-human trial exhibited adverse effects like diarrhea (2.3%), headache (1.3%), vomiting (1.3%), nausea (1.3%), decreased renal function (3.7%), hypotension (1.0%), and hypertension (2.3%) (2). The adverse effects of plazomicin were comparable to those of meropenem in the evaluation of plazomicin in a cUTI (EPIC) clinical trial. More than 1% of the patients reported adverse events including headache, hypertension, nausea, diarrhea, vomiting, and hypotension were similar in the meropenem trial (38). Adverse events related to vestibular and cochlear function were observed in only one patient in each treatment trial. Adverse renal effects, as evaluated by a blinded review, included a decrease in creatinine clearance and an increase in blood creatinine levels, renal failure, acute kidney injury, and chronic kidney disease. Renal impairment occurred in four (1.3%) patients in the meropenem trial versus 11 (3.6%) in the plazomicin trial. A serum creatinine level > 0.5 mg/dL were observed in 12 (4.0%) patients in the meropenem trial versus 21 (7.0%) in the plazomicin trial. The full recovery rate for the increased serum creatinine levels at the end of the IV therapy was observed in four of nine (44.4%) patients in the meropenem trial versus six of 11 (54.5%) in the plazomicin trial. At the last follow-up visit, the full recovery of renal function was observed in nine of nine (100%) patients in the meropenem trial versus nine of 11 (81.8%) in the plazomicin trial. In addition, increased serum creatinine levels were observed in a small number of patients (three of 297 patients in the meropenem trial versus 10 of 300 patients in the plazomicin trial) approximately one week after therapy completion (38). The incidence of nephrotoxicity was higher in patients with a plazomicin plasma concentrations > 3 μg/mL (36%, 10 of 28 patients) compared to those with plazomicin plasma concentrations < 3 μg /mL (5%, 11 of 215 patients) (35). The risk of neuromuscular blockade causing respiratory depression is related to AG antibiotics but has not been observed in plazomicin clinical trials. Proper precautions must be taken when plazomicin is administered into a patient with neuromuscular disorders, such as myasthenia gravis, or in those who use neuromuscular blockers, such as succinylcholine, as it may cause a delay in the recovery rate of neuromuscular function (38). The likelihood of plazomicin interacting with other drugs was evaluated following in vitro testing against a panel of drug transporters and drug-metabolizing enzymes. The main drug transporters, such as p-glycoproteins, do not interact with plazomicin (47, 50, 52, 53, (54).
CONCLUSION
Plazomicin is effective against a broad range of Enterobacteriaceae species. Plazomicin exhibited better efficacy than meropenem which is used to treat adult cUTIs including pyelonephritis. Plazomicin has a few dosing advantages over comparative drugs, including β-lactamase/β-lactam inhibitors, which are effective against gram-negative MDR organisms. Plazomicin is especially beneficial for patients who are allergic to other antibiotics, or at risk of resistant Enterobacteriaceae infections. Plazomicin was administered through an IV once daily for 30 min. This plazomicin administration schedule is useful for treating hospitalized patients and for patients receiving outpatient parenteral antibiotic therapy. Therefore, plazomicin is a promising new antibiotics effective against multiple antibiotic-resistant bacteria.