INTRODUCTION
Arthropod-borne viruses (arboviruses), particularly mosquito-borne viruses, are an emerging global public health problem. Each year there are 390 million cases of arbovirus infection, including asymptomatic infection, worldwide. The risk of infection by these viruses continues to increase and is a threat to public health (1). Dengue virus (DENV) and chikungunya virus (CHIKV) are arboviruses that have similar transmission vectors, geographical distribution, and seasonal correlation. However, the progression of these two infections leads to radically different disease outcomes. Acute arthritis and rash are the prominent symptoms of CHIKV infection, whereas some cases of dengue fever are characterized by plasma leakage that causes bleeding and shock (2, 3). Thus, there is a need to develop differential diagnostic methods for detecting CHIKV and DENV infections to aid patient care and epidemiological characterization and improve disease management (4).
The commercial diagnostic methods for DENV and CHIKV infections include isolation of the viruses from susceptible cells, followed by real-time polymerase chain reaction (PCR) analysis. However, these methodologies require sophisticated and expensive equipment (5). The other widely used diagnostic method is the serological test (IgM and IgG enzyme-linked immunosorbent assay [ELISA]), which is less sensitive than antigen detection. However, the serological test is not effective for virus detection in the first 5 to 6 days of infection. Time is an important factor for diagnosis and disease management (6); therefore, a rapid, simple, and reliable method is needed for the differential diagnosis of viral infections that exhibit similar symptoms.
CHIKV and DENV are enveloped viruses that have single-stranded positive-sense RNA genomes. The size of the viral RNAs is approximately 11 kb, which encode both structural and nonstructural proteins. The envelope protein (EP) is a structural glycoprotein present on the surface of the virus and mediates the virus entry into susceptible cells during infection. Additionally, EP is the main target of the host immune response against the virus (7). The EP can be a potential diagnostic marker for the improved clinical diagnosis of these viral infections (8, 9).
Ficolins are members of the defense collagen family of proteins that recognize a variety of invading pathogens and activate the complement lectin pathway in the human innate immune system (10). Ficolins are oligomeric proteins comprised of trimeric subunits. They also contain collagen-like and fibrinogen-related recognition domains that specifically bind to the pathogen-associated N-linked glycan (11). There are three types of human ficolins: ficolin-1, ficolin-2, and ficolin-3. Ficolin-1 recognizes the N-linked glycan of microbial carbohydrates through its fibrinogen-like (FBG) domain and activates the complement lectin pathway (12, 13). Recently, several reports have demonstrated that ficolin-1 binds to the viral EP and bacterial membrane and activates the complement system to initiate an antimicrobial response (11, 14, 15).
In this study, we developed a novel multiplex diagnostic system based on the lateral flow assay (LFA) using ficolin-1 and two-color latex beads labeled with antibodies to detect DENV and CHIKV on a single strip. Ficolin-1 was used as the capturing agent at the test line; red latex beads conjugated with anti-DENV monoclonal antibody (mAb) and blue latex beads conjugated with anti-CHIKV mAb were used as the detecting agents in the strip test. Accumulation of the virus–colored detecting agent complex at the test line was based on the virus capture by ficolin-1. The differential diagnosis of DENV-2 and CHIKV can be achieved by observing the color of the test line. The limit of detection (LOD) by naked-eye observation in our multiplex LFA for the viral EP was 25 nM and that for the whole virus was 1 ´ 106 TCID50/mL per strip. To the best of our knowledge, this is the first study to simultaneously detect and differentiate DENV and CHIKV in a single test line with ficolin-1 on a single strip. This LFA will be a powerful tool as a multiplex platform for the rapid differential diagnosis of DENV and CHIKV and can have clinical applications.
MATERIALS AND METHODS
Materials
The streptavidin binding peptide (SBP)-tagged ficolin-1, His-tagged envelope protein (EP) of DENV and CHIKV were expressed in the insect cells using the recombinant baculoviruses and were purified by affinity chromatography as described previously (11, 16). We used the following EPs that had deletions in the C-terminal transmembrane (TM) domain: CHIKV envelope 1 protein (E1P) ΔTM, CHIKV envelope 2 protein (E2P)ΔTM, DENV-1 EPΔTM, DENV-2 EPΔTM, DENV-3 EPΔTM, and DENV-4 EPΔTM. The EPs with TM-domain deletion were used to enhance the secretion of EPs. The anti-CHIKV monoclonal antibody (mAb) was generated in our laboratory and the anti-DENV mAb was purchased from Abcam Inc. (USA). The anti-DENV mAb was reactive with DENV serotype-1, -2, -3, and -4. The nitrocellulose (NC) membrane and laminated cards were obtained from Millipore (USA). The absorbent pad and the sample pad were procured from Millipore (USA). The blue and red latex beads were supplied by Expedeon (UK). The goat anti-mouse IgG antibody (Ab) and other chemicals were purchased from Sigma-Aldrich (USA).
Non-trypsinized virus culture and virus titration
DENV-2 and CHIKV were provided by the BioNano Health Guard Research Center (Daejeon, Korea). Non-trypsinized virus strains were propagated in Madin-Darby canine kidney (MDCK) cells to make working stocks of the virus. Virus infection should be performed on an 80 ~ 90% confluent. Confluent MDCK cells were infected with virus at multiplicities of infection (MOI) of 0.01 ~ 0.1 and incubated for 1 hour at 37°C in a humidified 5% CO2 atmosphere. The culture fluid was removed, and the cells were incubated with the infection medium (without TPCK-trypsin and FBS). Virus stocks were kept in aliquots at -70°C.
The virus was titrated in MDCK cells to determine the 50% tissue culture infective dose
(TCID50) by the Reed and Muench method (17). MDCK cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 5% fetal bovine serum (FBS).
Binding assay for ficolin-1 and viral EP
Analysis of ficolin-1 binding EP using ELISA
The binding of ficolin-1 to the EPs of DENV and CHIKV was determined using a ficolin-1-EP-capture-ELISA (18). Briefly, the microtiter plates were coated with bovine serum albumin (BSA) or purified EP (1 μg/mL in carbonate buffer 50 mM Na2CO3, 50 mM NaHCO3, pH 9.0) at 4°C overnight. The nonspecific binding sites in each well were blocked with 2% BSA in phosphate-buffered saline (PBS, pH 7.4) before incubation with ficolin-1. The plates were incubated with the purified SBP-tagged ficolin-1 (10 μM) in binding buffer (20 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.05% Tween-20, 0.1% (w/v) BSA, 10 mM MgCl2, and 10 mM CaCl2) for 1 h at 25°C. The plate was washed and incubated with HRP-conjugated streptavidin (Thermo Fisher Scientific, USA). The plate was subjected to three washing steps using PBST (0.05% Tween-20 in PBS). The signal was developed using 3,3′,5,5′-tetramethylbenzidine (TMB) solution (Sigma, USA), and the reaction was terminated by the addition of a stop solution (Abcam Inc., USA). The absorbance of the sample was measured using a microplate reader at 450 nm. The plate was subjected to five washes with PBST between each step.
Analysis of ficolin-1 binding EP using immunoprecipitation (IP)
The SBP-tagged ficolin-1 (10 μM) was incubated with the streptavidin magnetic beads. The ficolin-1-coated magnetic particles were blocked using 2% BSA and were subjected to IP using purified His-tagged EP (5 μM) in binding buffer. After five washes, the samples were added to the SDS sample buffer and resolved by 12-4% gradient SDS-PAGE (Invitrogen, USA). Western blotting was performed using 1 μg/mL rabbit anti-His polyclonal antibody and HRP-conjugated anti-rabbit IgG antibody (BIO-RAD, USA).
Evaluating the interaction of ficolin-1 and viruses with the target by overlay assay
Two microliters of DENV-2 (1 × 107 TCIC50/mL) and CHIKV (1 × 107 TCIC50/mL) were spotted onto the Hybond C-extra NC membrane (BIO-RAD, USA) next to each other. The membrane was blocked for 1 h at 25°C in tris-buffered saline containing 0.05% Tween-20, 10 mM CaCl2, and 5% skim milk. The membrane was then incubated with SBP-tagged ficolin-1 (10 mM) at 4°C overnight. The membrane was washed three times for 20 min at each wash. The ficolin-1-overlaid membrane was incubated with HRP-conjugated streptavidin for 1 h at 25°C. After the washing steps, the interaction was detected by measuring the enhanced chemiluminescence (ECL kit, Thermo Fisher Scientific, USA).
Assembly of strip test
The test system in this study included an activation solution, a detection agent solution, and the test strips (19, 20). The activation solution was a mixture of 25 mM Tris-HCl, pH 7.4, 100 mM NaCl, 10 mM CaCl2, 10 mM MgCl2, and 0.1% v/v Tween-20 (Thermo Fisher Scientific, USA). The latex beads were covalently conjugated to the lysine residues on the antibody. The detection agent solution contained colored latex beads conjugated with anti-CHIKV mAb and anti-DENV mAb in the activation solution. These antibodies were used to detect the viral EP. The strip consisted of plastic-based components: a sample pad, an NC membrane, and an absorption pad. A piece of the NC membrane was used to immobilize the proteins in distinct zones using a dispenser (DCI100; Zeta Corporation, Korea). The capturing agent, SBP-tagged ficolin-1 (10 μM) was used to form the test line, the anti-mouse IgG antibody (0.03 mg/mL) was used to form the control line, and the membrane was air-dried. The red latex beads conjugated with anti-DENV mAb and the blue latex beads conjugated with anti-CHIKV mAb were used for the detection of virus (21). The latex bead labeled with the antibodies was blocked with ficolin-1 (1 μM) as the binding of IgG Ab and ficolin-1 was weak.
Test strip analysis
The EP and virus sample were mixed with 50 µL of the detection agent solution and incubated for 5 min to form the target protein-antibody complex. The mixture was then pipetted onto a test strip sample pad. The mixture was allowed to migrate along the strip membrane by capillary action. During the migration across the NC membrane, the target protein-antibody complex was able to interact with the capturing agent, ficolin-1. The EP was tested at various concentrations (100, 75, 50, 25, and 10 nM). The whole virus was tested using a viral load of 1× 107, 1× 106, 1 × 105, 1 × 104, and 1 × 103 TCID50/mL. We used PBST with human serum albumin as the negative control. All colorimetric signals were generated using the colored latex beads, which are red or blue. The limit of detection (the lowest concentrations of EPs or virus that can be distinguished from blank samples) were defined for the two-color assay from the spiked buffer test, respectively. The detection limit was determined through repeated experiments on the signal intensity value of the test line using statistical analysis.
Statistical analysis
The signal intensity of the test lines was quantified using a custom script in Fujifilm multi-gauge program (Fuji photo film Co, Ltd. Japan). A region was drawn around the test line of interest, and the average pixel intensity inside this test region was computed. This value was then background-subtracted using the average pixel intensity inside a local background region and the normalized pixel intensity of the spot was determined. The data sets were statistically analyzed using Origin 6 software (OriginLab, USA).
RESULTS
Detection scheme of multiplex LFA using ficolin-1
To detect DENV and CHIKV on a single strip, we developed a novel multiplex diagnostic system based on LFA using ficolin-1 and antibody-labeled two-color latex beads. This method is a differential diagnostic technique for detecting DENV and CHIKV by observing the color indicated at the test line on a single strip. The principle of our multiplex LFA is schematically illustrated in Fig. 1. Similar to the other commonly used strip sensors, the multiplex LFA strip sensor consists of a test line and a control line (Fig. 1(a)). Ficolin-1 and anti-mouse immunoglobulin G (IgG) antibody (Ab) were immobilized on nitrocellulose (NC) membrane at the test and control lines, respectively. We used anti-DENV mAb-conjugated red latex beads and anti-CHIKV mAb-conjugated blue latex beads as the detecting agents in the strip test. When the mixture of sample and detecting agents is loaded on the sample pad, the solution moves horizontally through the sample pad and NC membrane (Fig. 1(b)). Initially, the target protein–colored detecting agent complex in the samples was captured by ficolin-1 on the test line and the remaining free detecting agents were bound to the anti-mouse IgG Ab-fixed control line. The accumulation of the complex produced a visible test line. DENV-2 and CHIKV can be differentially diagnosed by observing the color of the test line. As shown in Figs. 1(c)-(d), red color is observed in the presence of DENV-2, whereas blue color is observed in the presence of CHIKV.
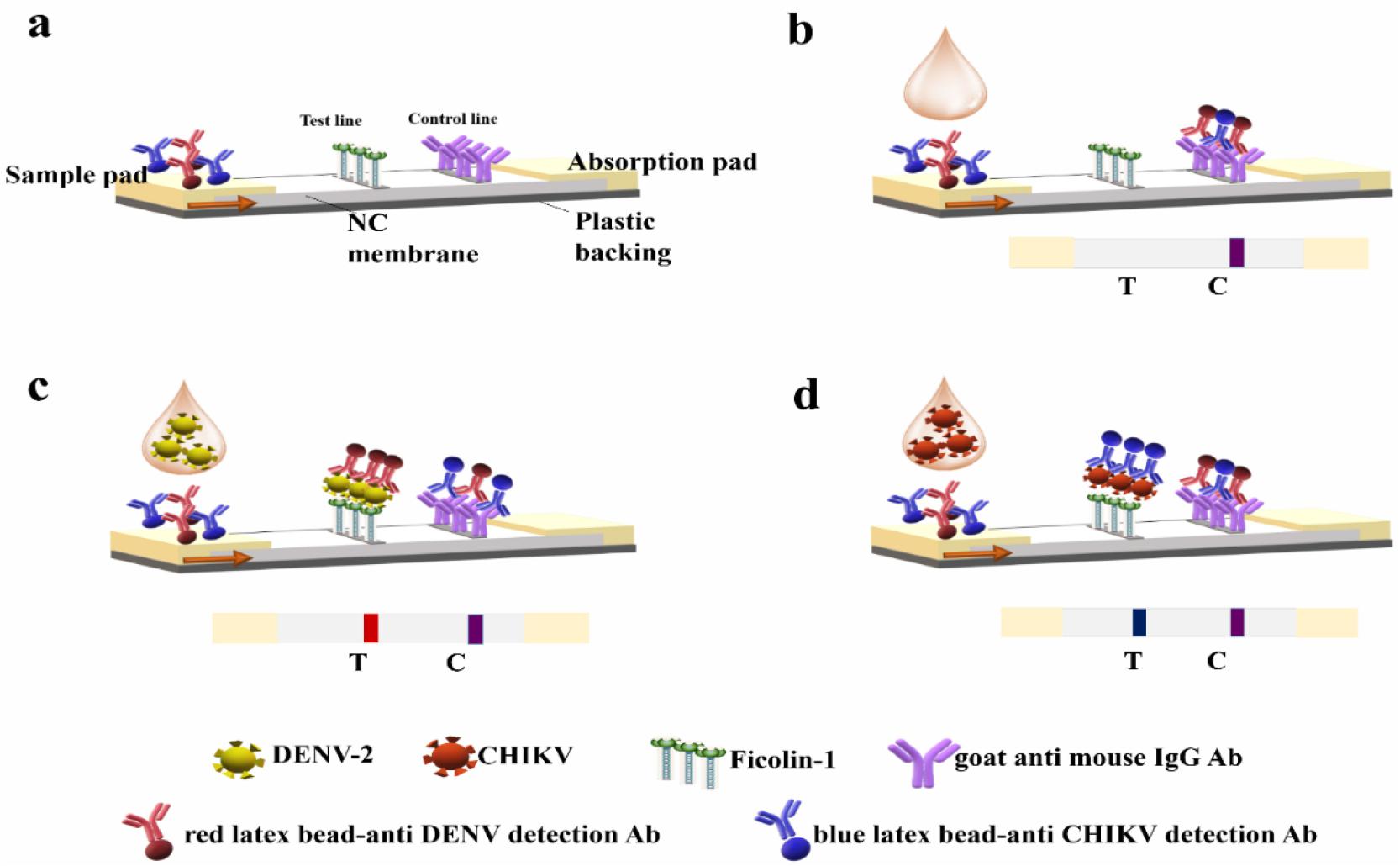
Fig. 1
Schematic illustration of the ficolin-1-based multiplex lateral flow assay (LFA) system.
(a) One test line and one control line are immobilized on the nitrocellulose membrane (NC) membrane. The test line contains ficolin-1 to capture the target pathogen, whereas the control line contains the goat anti-mouse immunoglobulin G (IgG) antibody (Ab) to capture the mouse antibody-conjugated detecting agents in the sample flow. (b-d) The mixture of virus samples and detecting agents are applied to the sample pad. During migration, the virus–detecting agent complex is captured by ficolin-1 at the test line. Accumulation of the complex produced a visible colored test line. The free detecting agents are captured by the anti-mouse IgG Ab at the control line. Red color represents DENV-2 in sample (c) and blue color indicates CHIKV (d).
Ficolin-1 binds to viral EP
Among the members of the ficolin family of innate immune recognition proteins, ficolin-1 uniquely binds to the N-linked glycans of pathogens. The serum ficolin-1 appears to be a pattern recognition molecule that can sense several microbes of clinical interest by binding to the N-linked glycan structure of the surface proteins.
To confirm whether ficolin-1 recognizes and binds to the EP of DENV and CHIKV, various protein binding assays were performed using the recombinant ficolin-1 and the glycosylated EPs that were derived from the insect cells. For the ELISA, we first used various recombinant EPs with a deleted C-terminal transmembrane (TM) domain: CHIKV envelope 1 protein (E1P)ΔTM, CHIKV envelope 2 protein (E2P)ΔTM, DENV-1 EPΔTM, DENV-2 EPΔTM, DENV-3 EPΔTM, and DENV-4 EPΔTM. The ELISA analysis demonstrated that ficolin-1 binds to all the EPs from the DENV serotype-1, -2, -3, -4, and CHIKV (Fig. 2(a)). As DENV-2 is the major circulating serotype and CHIKV E2P is a major diagnostic target, we used the DENV-2 EPΔTM and CHIKV E2PΔTM for further analysis. The physical interaction between ficolin-1 and EPs was also determined by the immunoprecipitation (IP) assay. The EPs of DENV-2 or CHIKV were incubated with the streptavidin magnetic bead-SBP (streptavidin binding peptide) tagged-ficolin-1 complex or streptavidin magnetic bead-BSA complex. The magnetic beads were pulled down and were subjected to SDS-PAGE and western blotting. The western blotting analysis confirmed that ficolin-1 recognizes the EP (Fig. 2(b)).
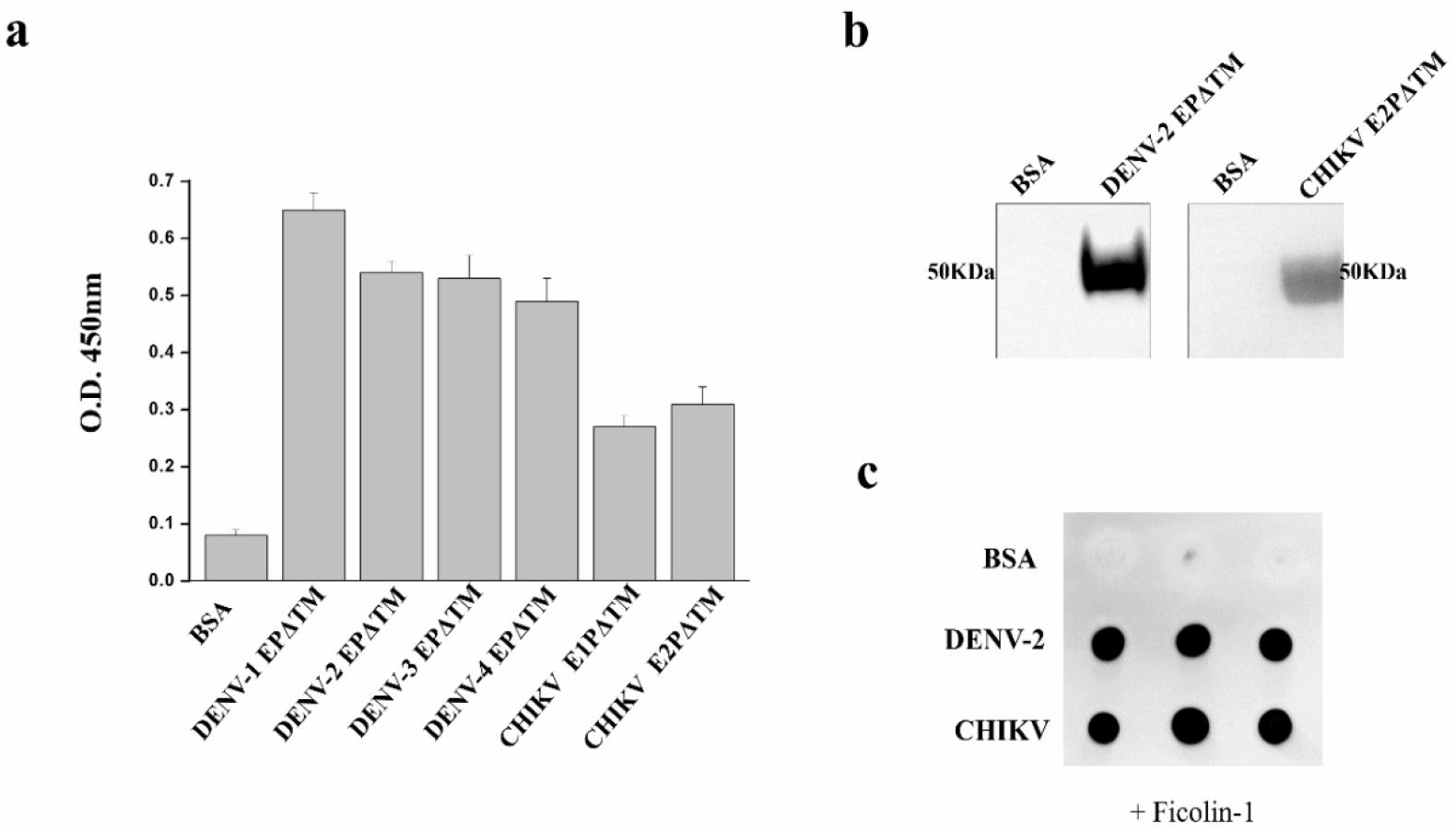
Fig. 2
Characterization of the interaction between ficolin-1 and the envelope proteins (EPs) of DENV and CHIKV.
Enzyme-linked immunosorbent assay (ELISA). The EPs from DENV and CHIKV bind directly to ficolin-1. The purified EPs were coated on a microtiter plate and incubated with ficolin-1 for ELISA. (b) Immunoprecipitation (IP) assay. DENV-2 EPΔTM, CHIKV E2PΔTM, or control BSA were incubated with purified ficolin-1. Following IP with streptavidin magnetic beads western blotting was performed. The immunoprecipitated samples were subjected to SDS-PAGE and probed with a mouse anti-His monoclonal antibody (mAb). (c) Virus-protein overlay assay. The virus was spotted onto the nitrocellulose (NC) membrane and incubated with purified streptavidin binding peptide (SBP) tagged-ficolin-1. After three washes, the bound ficolin-1 was treated with a specific streptavidin-horse radish peroxidase (HRP) and detected using enhanced chemiluminescence (ECL).
We used a virus-protein overlay assay to determine the binding of ficolin-1 to the whole virus. The DENV-2 and CHIKV were spotted onto the NC membrane and incubated with ficolin-1. The binding of ficolin-1 to the virus after washing the membrane was determined by the chemiluminescent signal. A robust signal was only detected at the virus spots (Fig. 2(c)). This suggested that the DENV-2 and CHIKV bind to ficolin-1.
Optimization of LFA
To optimize the performance of multiplex LFA, LFA was evaluated using a dilution series of the DENV-2 EPΔTM, CHIKV E2PΔTM, and the whole virus. The assay response graph and the representative image of the test line at each concentration for EPs and the whole virus are shown in Figs. 3 and 4. The LOD for the detection of EP using ficolin-1 as the capturing agent was 10 nM (Fig. 3). Furthermore, we confirmed the detection of whole DENV-2 and CHIKV. The LOD for the naked-eye detection of whole virus was determined to be 1 × 104 TCIC50/mL. The quantification of the band intensities revealed a LOD of 1 × 103 TCIC50/mL (Fig. 4). For the multiplex detection, we also evaluated the cross-reactivity between DENV-2 and CHIKV. We did not observe any cross-reactivity in this strip (Fig. 4). These results suggested that ficolin-1 strips may be useful for the simultaneous diagnosis of DENV-2 and CHIKV.
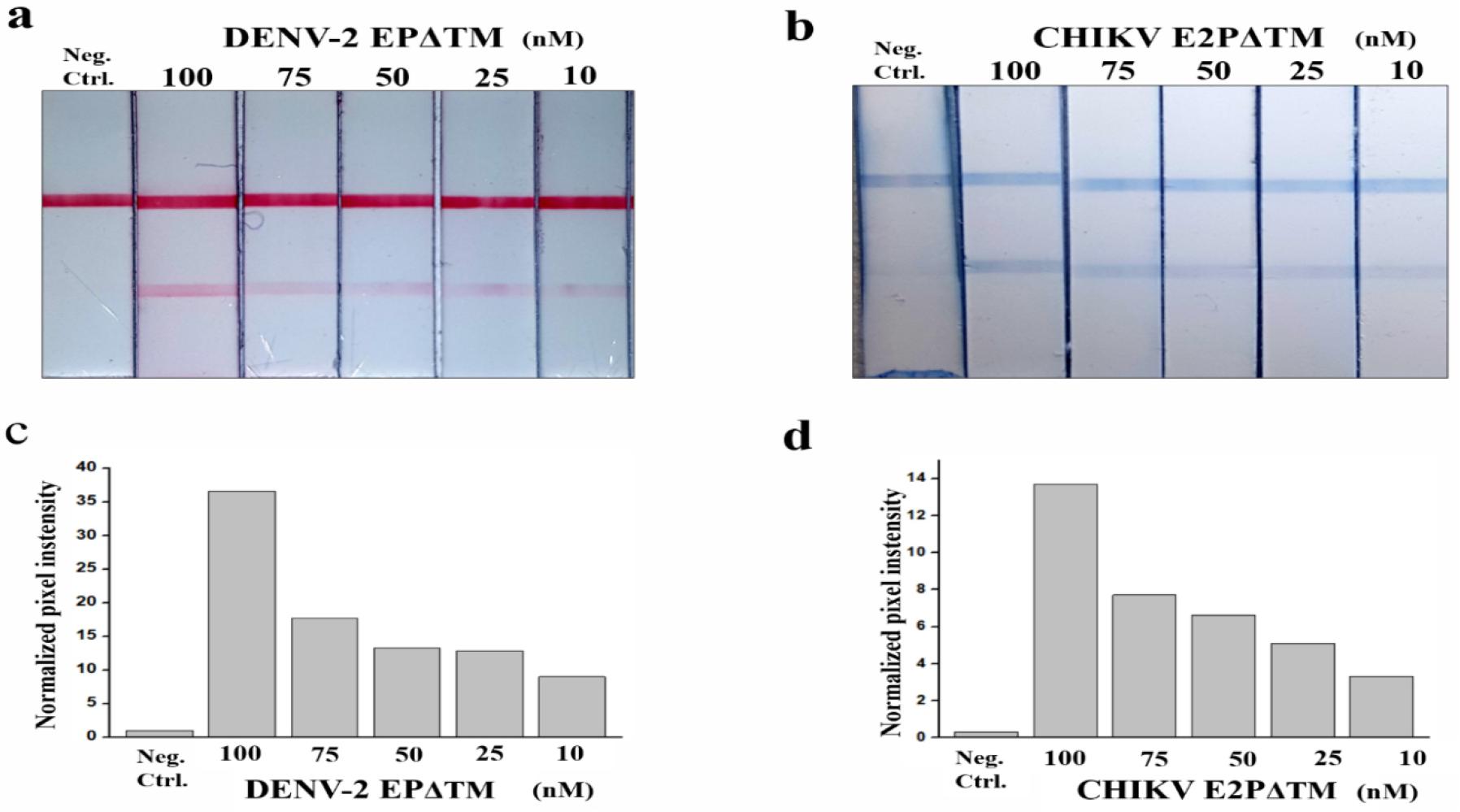
Fig. 3
Multiplex lateral flow assay (LFA) using ficolin-1 for the envelope protein (EP) detection of DENV and CHIKV.
(a, b) The image of LFA with different concentrations of DENV-2 EPΔTM and CHIKV E2PΔTM. (c, d) The intensity of red and blue colors in the control and test line was converted to signal peak intensity using image analysis software. The relative peak intensity represents the peak intensity ratio of the test to control line and is used as the detection signal to estimate the assay performance. Neg. Ctrl, negative control.
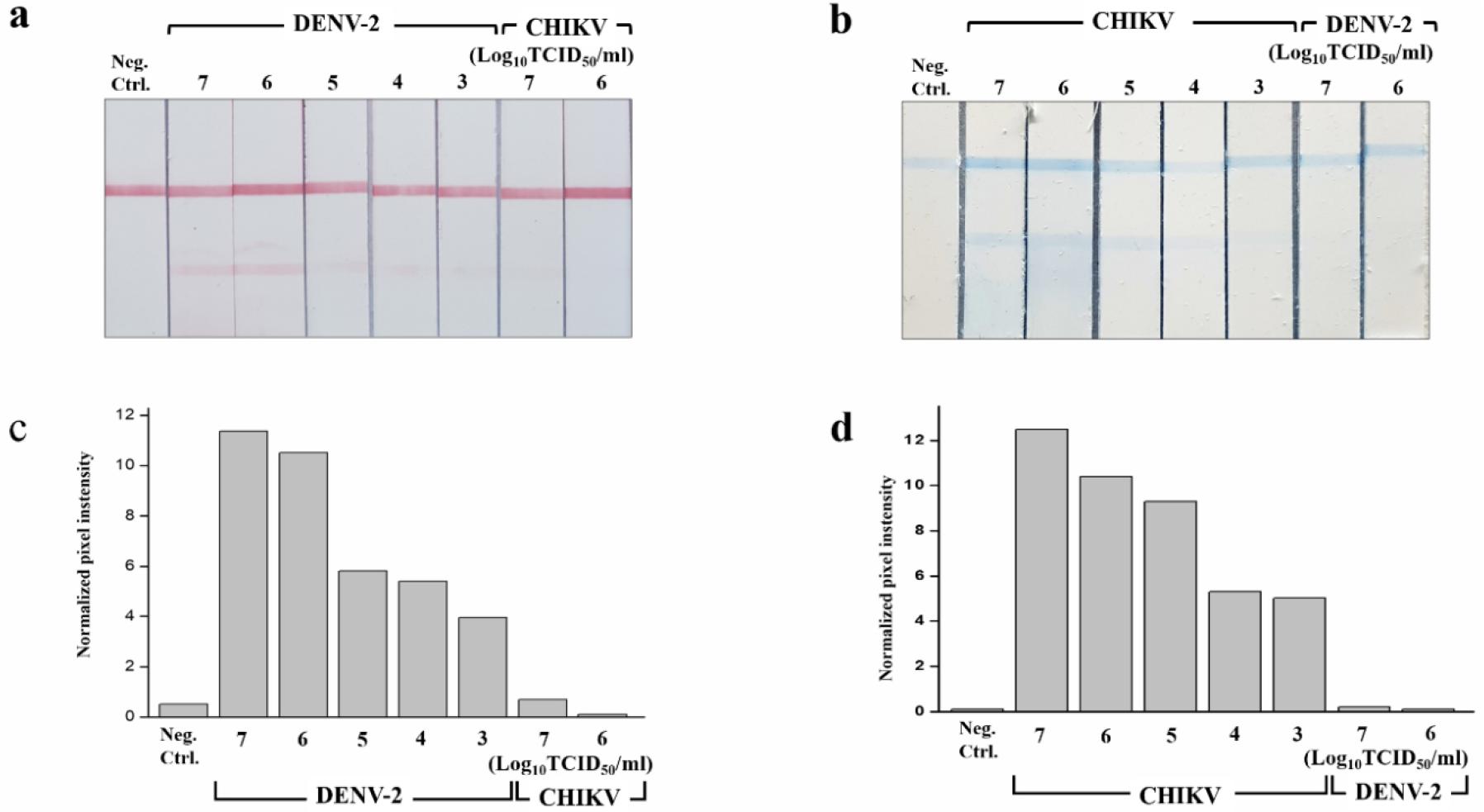
Fig. 4
Multiplex lateral flow assay (LFA) using ficolin-1 for the whole virus detection of DENV-2 and CHIKV.
(a, b) The image of LFA with different concentrations of DENV-2 and CHIKV. (c, d) Quantification of the band intensities at the strip test line. The intensity of the bands was quantified using image processing software and was expressed as the normalized pixel intensity values for each virus. Neg. Ctrl, negative control.
Multiplex LFA for simultaneous detection of DENV-2 and CHIKV
For the simultaneous detection of DENV-2 and CHIKV with multiplex LFA, we demonstrated the performance of multiple LFA with two-color latex beads on one test line of a single strip. When the sample contained CHIKV E2PΔTM, only blue color was observed on the test line. The blue color on the test line indicated the binding between blue latex beads–anti-CHIKV mAb detecting agent and CHIKV E2PΔTM in the sample, which in turn was captured by ficolin-1 on the test line. Conversely, when the sample contained DENV-2 EPΔTM, red color was observed. This correlated with the accumulation of red latex bead–anti-DENV mAb detecting agent on the test line (Figs. 5(a)-5(c)). Further, we mixed the two-color detecting agents with one target and performed LFA. We obtained a strong red or blue signal when the minimum target concentration was 25 nM. Next, we also tested the detection of whole virus on the strip. The two-color multiplex LFA developed distinct red/blue colors that were visible to the naked eye when the concentrations of DENV-2 and CHIKV were above 1 × 106 TCID50/mL (Figs. 5(b)-5(d)). In comparison to the commercial rapid diagnostic tests (RDTs) for duplex anti-CHIKV IgG/IgM detection that requires two separate test regions, our assay is expected to achieve the same level of multiplexing on just a single test line. This could lead to the reduction in the strip dimensions, material cost, and required sample volume.
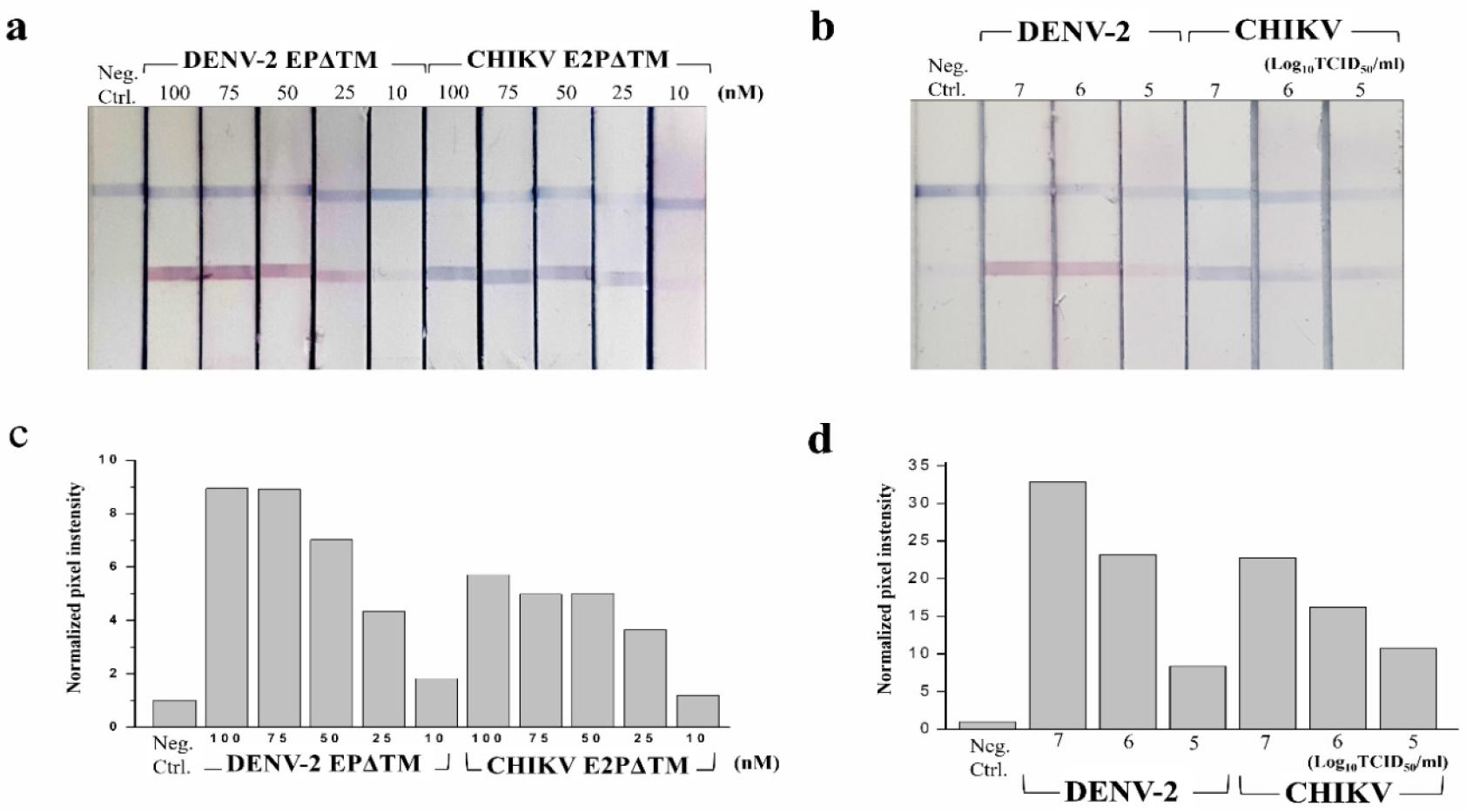
Fig. 5
Multiplex lateral flow assay (LFA) using ficolin-1 for the simultaneous detection of DENV-2 and CHIKV. (a) Image of ficolin-1-based LFA with recombinant envelope proteins (Eps) of DENV-2 and CHIKV. (b) Image of ficolin-1-based LFA with whole DENV-2 and CHIKV. (c-d) Quantification of the band intensities at the strip test line. The normalized pixel intensity was quantified using Image software. The intensity values were calculated relative to the control line intensity. Neg. Ctrl, negative control.
DISCUSSION
DENV and CHIKV are the most common mosquito-borne viral diseases with similar symptoms and co-circulation, but the progression of these two infections leads to different disease outcomes. Thus, these infectious diseases need to be individually diagnosed by identifying the causative infectious agent at an early stage. While LFA has been used widely for rapid diagnosis due to its simplicity, rapidity, and cost-effectiveness, its sensitivity is often limited by the use of optimal antibody-antigen pairs and by colorimetric detection.
In this study, we developed a novel multiplex diagnostic system based on the LFA using ficolin-1 and two-color latex beads labeled with antibodies to detect DENV and CHIKV in a single strip. The assay was first employed ficolin-1 as a capture agent instead of antibodies to the viral EP. Our results also demonstrated that DENV-2 and CHIKV can be simultaneously detected by observing the color of the detecting agent.
The LFA analysis is a recognized POCT (point–of–care testing) technique. By the method for preparing the colored latex particles, the process is simple, the cost is reduced, and the prepared colored latex particles have rich colors, controllable color shade, and clean surfaces, and do not change morphology. In addition, conclusions similar to those of ELISA can be observed in a shorter period, reducing ambient contamination from hazardous samples.
In this experiment, the intensity of the band that appeared on the strip was calculated using an image program and this was determined as the detection limit. The LOD by naked-eye observation in our multiplex LFA for the viral EPs was 25 nM and that for the whole virus was 1 ´ 106 TCID50/mL per strip. Unfortunately, infected patient samples were difficult to obtain, only viral Eps and cultured viruses were used in the test. Thus, further studies on the clinical samples are required.
In conclusion, our LFA using ficolin-1 can be a useful tool for the detection of viruses in routine laboratory testing. Thus, this multiplex LFA system using ficolin-1 can simultaneously detect viruses easily and rapidly without expensive equipment and complicated protocols.




