INTRODUCTION
Inflammation is a process that protects the host from infection by various microorganisms such as viruses, bacteria, or other infectious microorganisms (1, 2) and can be classified as acute or chronic inflammation. Acute inflammation is a short- term protective response at a specific location where the problem exists. Chronic inflammation is associated with several diseases and has long-term effects throughout the body (3). Anti-inflammatory effects are mediated through the regulatory action of several cytokines, such as interleukins (ILs) and tumor necrosis factor (TNF), and non-cytokine molecules, such as prostaglandin E2 (PGE2) (3, 4). The most common anti-inflammatory agents are steroids, nonsteroidal anti-inflammatory drugs (NSAIDs), and immunosuppressants. However, all drugs cause adverse effects and complications that must be balanced through proper treatment management (5).
NSAIDs are commonly used worldwide (6). NSAIDs are classified based on their mechanisms of action and chemical structures. Some well-known classes are salicylate derivatives (aspirin, diflunisal), alkanones, propionic acid derivatives (naproxen and ibuprofen), anthranilic acid derivatives, indole derivatives (sulindac), and aniline derivatives (7). According to the mechanism of action, NSAIDs can be both selective and non-selective and inhibit the action of the cyclooxygenase (COX) enzyme (6, 8).
Many recent studies examined the alternative use of natural anti-inflammatory agents instead of synthetic anti-inflammatory agents because of their adverse reactions (6), showing that natural anti-inflammatory agents and supplements could provide an alternative to synthetic NSAIDs. The purpose of this study is to inform the general public about the beneficial aspects of natural anti-inflammatory agents and explain the differences between synthetic and natural anti-inflammatory agents; it discusses how natural anti-inflammatory agents and supplements help reduce inflammation without serious harmful effects (9).
NATURAL POLYSACCHARIDES
Polysaccharides are polymeric carbohydrates made up of sugar units that are linked by glycosidic bonds (10). Naturally extracted polysaccharides have attracted attention because of their health-promoting properties and ability to reduce inflammation (10, 11).
Natural polysaccharides are classified into three main categories: higher plant polysaccharides (e.g., gums, pectin, and inulin), microbial exopolysaccharides (e.g., dextran, levan, gellan, and hyaluronic acid), and marine polysaccharides (e.g., alginate, carrageenans, and chitosan). Generally, natural polysaccharides are extracted and isolated from crude polysaccharides through deproteinization and decoloration (11). Currently, chemical methods are used to isolate and extract natural polysaccharides; chromatography, spectroscopy, and nuclear magnetic resonance (NMR) techniques have been used for the structural analysis of natural polysaccharides (11, 12). The mechanisms of action of natural polysaccharides are listed in Table 1(13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33).
Table 1.
An insight into the anti-inflammatory mechanisms of selected natural polysaccharides
| No. | Name of the compound | Source | Origin | Involved mechanism | Ref. |
|---|---|---|---|---|---|
| 1 | CCPSn (Cipangopaludina chinensis polysaccharides) | Flesh of Cipangopaludina chinensis | Snail | Suppress the activity of PGE2 and NO, prevent COX2 and iNOS expression and further reduces the secretion of inflammatory cytokines | (13) |
| 2 | ASPP (alkali soluble polysaccharides) | Purple sweet potato | Plant | reduce IL-6, NO and TNF-α level while enhance the level of IL-10 in inflammatory areas | (14) |
| 3 | WPSPP-1 (Water soluble polysaccharides) | Purple sweet potato | Plant | Lowers the level of IL-6, IL-10 and TNF-α to reduce inflammation | (15) |
| 4 | PEPS (Pleurotus eryngii polysaccharides) | Pleurotus eryngii | Mushroom | Lower the secretion of inflammatory mediators | (16) |
| 5 | CCP (Crude polysaccharides) | Sargassum horneri | Marine algae | Inhibit the activating mechanism of MPAK and NF-κB signaling cascade and also block the regulation of cytokines | (17) |
| 6 | ALP-1 (Arctium lappa polysaccharides) | Arctium lappa | Chinese herbs | Greatly enhance the level of Firmicutes, Ruminococcaceae and Lactobacillus while remarkably decrease the activity of Alcaligenaceae, Proteobacteria and Staphylococcus, significantly decrease the action of cytokines, reduce secretion of IL-1β from NLRP3 and inhibit VCAM-1 expression | (18) |
| 7 | GLPss58 (Sulfated Ganoderma lucidum polysaccharides) | Ganoderma lucidum | Chinese herbs | Increase the serum level of IgA and IgG, increase the receptor expression of poly-Ig in small intestine and enhance IL-1, IL-6 and TNF-α level | (19) |
| 8 | PJ-1 (panacis japonica polysaccharides) | Extracts of Rhizoma panacis japonica | Herbs | Exert anti-inflammatory action by activating the signaling pathway of STAT3 and by inhibiting the action of two major types of inflammatory cytokines; IL-1β and TNF-α | (20) |
| 9 | LEP (Lycium europaeum polysaccharide) | Leaves of Lycium europaeum L. | Plant | By using the stabilizing effects of mast cell, it blocks the signaling pathway of histamine. Also impede the transcription of histidine gene and suppress the action of H1 receptor along with the inhibition of COX2 and LOX production | (21) |
| 10 | Fucoidans | Ecklonia cava | Seaweed | Inhibit COX2 and iNOS expression and suppress the inflammatory action of cytokines such as IL-1β IL-16 and TNF-α etc. | (22,23) |
| 11 | EP-1 (Hericium erinaceus polysaccharides) | Hericium erinaceus | Mushroom | Suppress the action of several oxidative stresses such as Malondialdehyde (MDA), NO, COX2 and TNF- α etc. | (24) |
| 12 | HCP (a water-soluble polysaccharide from Sarcodonas pratus) | Sarcodonas pratus | Mushroom | Down regulate the activity of inflammatory cytokines | (25) |
| 13 | DIP (Dictyophora indusiata Polysaccharide) | Dictyophora indusiata | Mushroom | Modulates the expression of TLR4 and inhibits the NF-kB signaling cascade | (26) |
| 14 | FVP (Flammuliana velutipes polysaccharides) | Flammuliana velutipes | Mushroom | Inhibit the phosphorylation activity of NF-kB DNA and damage the balance of cytokines | (27) |
| 15 | BMP (Blidingia minima Polysaccharides) | Blidingia minima | Seaweed | Modulate the PKB (Protein Kinase B) and NF-kB signaling pathway | (28) |
| 16 | APS (Astragalus polysaccharides) | Astragalus membranaceus | Chinese herbs | Decrease the production of inflammatory mediators and inhibit the action of iNOS gene by modulating NF-kB signaling cascade | (29) |
| 17 | DOP/EDOP (Dendrobium officinale Polysaccharides) | Dendrobium officinale | Chinese herbs | Reduce the activity of inflammatory cytokines | (30) |
| 18 | LBP (Lycium barbarum Polysaccharides) | Lycium barbarum | Chinese herbs | Reduce the production of MCP-1 (Monocyte Chemoattractant Protein) | (31) |
| 19 | CREP (Codonopsis pilosula Polysaccharides) | Codonopsis pilosula | Chinese herbs | Down regulate the action of interleukins and other cytokines | (32) |
| 20 | AIPetinc | Echinodontium tinctorium | Mushroom | Reduce nitric oxide synthesis along with TNF-α and other cytokines | (33) |
Natural polysaccharides affect the synthesis of cytokines and other regulators of inflammation such as TNF-α, IL-1, and IL-6, employing their anti-inflammatory mechanisms to inhibit cytokine release through modulating the nuclear factor-kappa B (NF-κB) signaling pathway (4, 10, 11, 12).
GREEN TEA
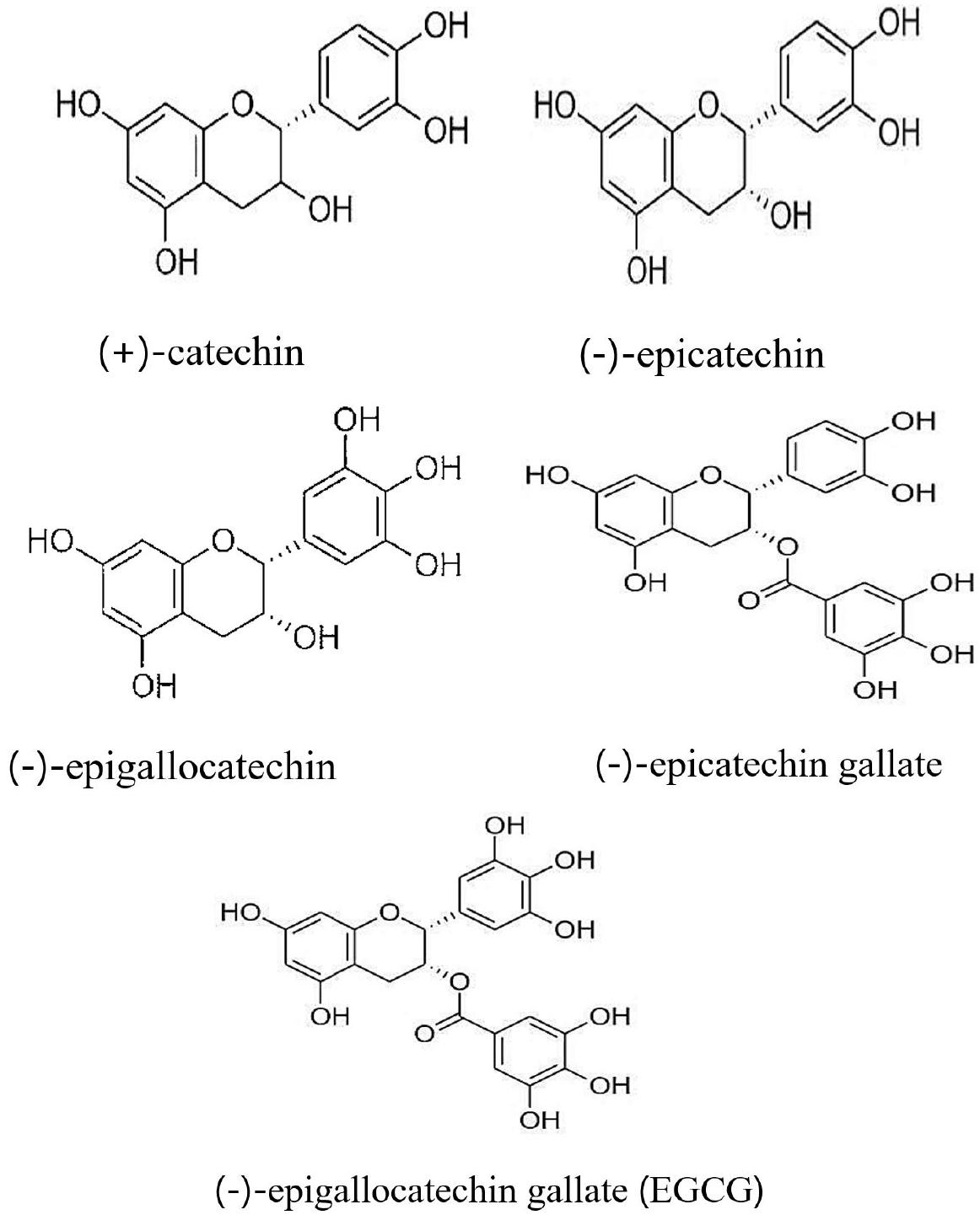
Studies have suggested that people who drink more green tea suffered less from the inflammatory diseases, and thus doctors assume that green tea (from Camellia sinensis plant) can be used to reduce inflammation (3, 34). The potential health benefits of Camellia sinensis plants have been widely studied. Green tea contains four main types of catechins which belong to the group of polyphenols: (-)-epicatechin, (-)-epigallocatechin, (-)-epicatechin, and (-)-epigallocatechin gallate (EGCG). These catechins can work both individually and conjunctionally. The biological effects of green tea are mainly exerted through polyphenol catechins (Fig. 1) in particular EGCG (34, 35, 36, 37). These elements can decrease the assembly of free radicals in the human body, protect cells and molecules from damage. Green tea catechins (GTCs) can affect inflammatory genes and proteins, such as TNF-α, matrix metalloproteinases, and IL, among others (34). EGCG reduces inflammation by inhibiting the inflammatory regulatory enzymes COX1 and COX2 (34). Green tea catechins can also reduce and suppress the migration capacity of nitric oxide (NO) synthesis, reactive oxygen species (ROS), and peroxynitrites. These results indicate that catechins have inflammation modulatory capacity (34).
Experiments on various animals have shown that green tea containing high catechin levels decreases the risk of galactosamine- induced hepatitis (34). Green tea beverages reduce aspartate transaminase and serum alanine transaminase levels, which are usually considered as biomarkers for liver health (34, 38). When mice were treated with EGCG, it significantly reduced the inflammatory response, oxidative stress, and liver injury (39). It has also been observed in bile-duct ligated rats that treatment with EGCG relieves and alleviates inflammation, liver necrosis, fibrosis and inhibits the genes expression participated in liver inflammation such as IL-1β, TNF-α, and transforming growth factor-β1 (39, 40).
Green tea and its EGCG component are also effective in treating cancer, vascular inflammation, liver injury, rheumatoid arthritis, osteoarthritis, ischemia, and brain disorders (34, 41). Pro-inflammatory cytokines play a vital role in the early stages of cancer development (42). The anticancer activity of green tea is associated with a decrease in the activity of the TNF-α gene and protein (34, 43, 44). EGCG inhibits the transcriptional activity of NF-κB in various cancer cells in the human body (34). Green tea and its EGCG component help reduce vascular inflammation by inhibiting the activity of TNF-α and IL-1β in the microvascular endothelial cells of the human brain (34, 45). TNF is a particular pleiotropic cytokine with a crucial role in inflammation, immune homeostasis, and host defense. It can induce different effects such as immune cell activation, apoptosis, necrosis, angiogenesis, differentiation, and cell migration. Above effects, processes are of related with tumor immune surveillance, and have a significant role in tumor development as well as tumor progression.
CURCUMIN
Curcumin, a bright yellow phytopolyphenol pigment found in the rhizomes of turmeric (Curcuma longa L.), has a significant effect in reducing inflammation (46). Curcumin exhibits anti-inflammatory action through the inhibition of some inflammatory regulatory enzymes such as COX2, lipoxygenase (LOX), inducible nitric oxide synthase (iNOS), ROS, and signal transducer and activator of transcription 3 (STAT3) (46, 47). These proteins are responsible for inflammation, and therefore, the inflammatory action will decrease as curcumin suppresses their activity (46).
Curcumin is also very effective in inhibiting PG synthesis (46, 48). PGs and other eicosanoids can cause serious disorders such as cancer. Several studies on humans and experimental animals have shown that excess PG is responsible for cell proliferation and tumor growth. Arachidonic acid is converted to PGs mainly by the COX enzyme. The COX enzyme has two isoforms, COX1 and COX2. Researchers have suggested that curcumin may block the expression of the COX2 enzyme. The dietary intake of curcumin may alter the activities of COX and LOX; hence, curcumin modifies the levels of PGE2. COX-1 and 2 catalyze the primary step in the PGs formation. In recent years, the role of COX in carcinogenesis has become more evident. They play a great role in apoptosis, angiogenesis, and invasion. These factors play a crucial role in the production of carcinogens. In addition, an increased level of COX-2 expression is found in cancer cells (49). iNOS is another important enzyme responsible for inflammation (46). This enzyme accelerates the production of NO, a carcinogenic agent that promotes neoplastic transformation and tumorigenesis. Curcumin stops the production of NO by inhibiting the expression of the iNOS enzyme (46). iNOS has been correlated with various types of tumors as well progression into metastatsis. iNOS is connected with cancers at a various sites of human body. Higher concentration of iNOS associated with the level of malignancy in hepatocellular carcinoma, gastric cancer, gynecological tumors, squamous cell carcinoma, melanoma and leukemia (50). In almost all types of cancers increased level of reactive oxygen species (ROS) was detected, where ROS also correlated with tumor development and progression. To detoxify from ROS, tumor cells also release antioxidant proteins (51). STAT3 expression is regulated in normal cells, however, in some malignancies, STAT3 is activated in a constitutive manner. Aberrant activation of STAT3 correlated with tumor metastasis take part in tumor cell proliferation, migration, angiogenesis, and invasion. It also leads to destroy of immune cells. STAT3 signaling control oncogenic pathway as well as facilitate immune evasion (52).
Generally, taking 0-3 mg/kg of curcumin per day is considered safe (47). Dietary supplements of curcumin elevate high-density lipoprotein cholesterol levels while significantly reducing low-density lipoprotein cholesterol levels (46, 47). Body cholesterol- related parameters (lipid profile and glucose profile body weight) can also change due to the regular administration of curcumin (47). Several in vitro and in vivo studies have confirmed that the health-promoting effects of curcumin can be attributed to its strong antioxidant and anti-inflammatory activities (46, 47). Curcumin is also effective against some chronic diseases such as diabetes, obesity, cancer, and neurological and autoimmune diseases (47, 53). Clinical trials have explored the therapeutic uses of curcumin supplementation in patients with inflammatory diseases such as arthritis, inflammatory bowel disease, peptic ulcer, and Helicobacter pylori infection (46, 47, 53). Thus, curcumin has an extensive role in biological activities and has therapeutic potential.
OMEGA-3 FATTY ACIDS
Omega-3 fatty acids (also known as n-3 fatty acids or omega-3 oils) are a type of polyunsaturated fatty acid of the omega series, meaning that they have double bonds on carbon-3 (54, 55). While these are essential nutrients for humans, our bodies cannot produce them. They are primarily found in oily fish such as salmon, bluefish, tuna, and halibut (54). These fatty acids are also found in canola, flaxseed, hemp, soybean, and walnut oils, soy, tofu, green leafy vegetables, venison, buffalo, among others (55).
Omega-3 fatty acids are usually three types: alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) (54, 55). EPA and DHA are the three main form of omega-3 fatty acids which are widely available as fish oil supplements and act as anti-inflammatory agents (54, 55). These fatty acids inhibit several inflammatory pathways such as the production of inflammatory regulators, leukocyte chemotaxis, leukocyte-endothelial adhesive interactions, production of eicosanoids, leukotrienes (LTs) and PGs, and T cell reactivity. EPA and DHA increase the levels of anti-inflammatory proteins and resolvins . EPA and DHA prevent the activation of NF-κB and reduce the expression of inflammatory genes (55). Clinical trials have shown that omega-3-fatty acids (n-3 polyunsaturated fatty acids) are beneficial in inflammatory bowel disease (IBD), asthma, and rheumatoid arthritis (RA) (54).
Marine polyunsaturated fatty acids are very effective in reducing heartburn and colonic damage. In almost all cases, the severity of these diseases is relative to the production of eicosanoids (PGs, LTs, etc.) derived from arachidonic acid. Administration of fish oil containing polyunsaturated fatty acids decreases the production of these eicosanoids. In some clinical trials, the use of fish oil in human IBD has been reported to be beneficial (55). Marine fish oil rich in polyunsaturated fatty acids improves the histopathology of the gut mucosa and patient clinical score and lowers the relapse rate (55). Eicosanoids (PGD2, PGE2, LTC4, and LTD4) derived from arachidonic acid also play an important role in various pulmonary inflammatory diseases and bronchoconstriction. PGE2, a type of eicosanoid, acts as a mediator in the development of asthma and plays an important role in developing the type 2 phenotype of T lymphocytes that can cause allergic inflammation (55). DHA helps to reduce lung inflammation and asthmatic conditions by inhibiting the efficiency of such eicosanoids (55).
Among the different types of inflammatory conditions, the effects of fish oil in the treatment of RA have been well examined. Animal experiments have demonstrated that marine polyunsaturated fatty acids (n-3 PUFAs) alleviate the severity of joint pain and arthritis (55, 56). The anti-inflammatory action of polyunsaturated fatty acids has been found in adult humans who consume at least 2 mg of omega-3 fatty acids per day (55). In RA, beneficial effects were observed at a dose of 3 mg/day of EPA with DHA for 12 days (55). Apart from these anti-inflammatory effects, omega-3 fatty acids are beneficial for mental health, depression, and schizophrenia.
COLCHICINE
Colchicine, a yellow compound extracted from the corns or seeds of colchicums (Colchicum autumnal), has been used to treat gout, Behcet’s disease (57), and joint swelling. Due to the side effects of anti-inflammatory painkillers, colchicine has been used as an alternative (57). Colchicine exerts its anti-inflammatory action by binding to tubulins and blocking the action and elongation of microtubule polymers, the key components of the cytoskeleton (58). Colchicine is capable to bind with tubulins which inhibiting the assembly and polymerization of microtubules. These microtubules are participated in different cellular processes such as cell migration, secretion of cytokine and chemokine, and regulation of ion channels. Tubulin-colchicine complexes are formed when colchicine binds to soluble tubulin. These complexes bind to the end of microtubules to prevent the microtubule polymer elongation (59). In inflamed tissues, it reduces the number of white blood cells, thereby breaking the inflammation cycle and reducing pain and swelling (58, 60).
Colchicine can also affect inflammatory pathology associated with arthritis and gout (58). Out of all anti-inflammatory drugs, including NSAIDs and steroids, colchicine is the most recommended drug for the treatment of gout and gouty arthritis (57). The pathology of gout and osteoarthritis involves the regulation of cytokines such as TNF-α and IL-1β. In vitro studies have illustrated that colchicine decreases the activity of inflammatory cytokines, alleviating gout and different types of arthritis including rheumatoid arthritis (57, 58). Colchicine inhibits various inflammatory pathways, such as superoxide production, adhesion, inflammatory activation, and production of proinflammatory cytokines. The main anti-inflammatory mechanisms are: colchicine decreased Nuclear Factor kappa-light-chain-enhancer of activated B cells (NF-κB) complex activity and inhibited immune response and inflammatory genes including NACHT-LRRPYD-containing protein 3 (NALRP3), IL-1, Pro-IL-1β, IL-6, and TNF. Colchicine inhibits the activation of NALRP3 and cysteine-dependent aspartate-directed proteases-1 (CASPASE-1). Moreover, Colchicine constrains the assembly, development, and elongation of microtubules and inhibits membrane-dependent functions (61, 62).
Cardiovascular diseases such as ischemic heart disease, hypertension, myocardial infarction, and hyperlipidemia can often manifest in patients with gout (57, 58). Gout itself acts as a risk factor for heart disease (58). Although colchicine is effective in reducing the activity of inflammatory cytokines, very few clinical studies have shown it to be beneficial in cardiovascular diseases (58). However, it has been shown that colchicine is safe and effective in the treatment of atherosclerosis, myocardial infarction, coronary disease, and familial Mediterranean fever (hereditary autoimmune inflammatory disorder) (57, 58). According to experts, colchicine and other anti-inflammatory drugs such as methotrexate and aspirin may provide remarkable pharmacological support for protecting the cardiovascular system (57). The position of colchicine as a therapeutic agent in medical science has been strengthened owing to its pleiotropic effects.
STINGLESS BEE HONEY
Stingless bees are small types of bees found in almost all continents of the world (63). It has been proven that stingless bee honey is more nutritious than normal honey (63). Depending on its geographical origin and botanical sources, the nutrient content of stingless bee honey may vary (64). Table 2 shows the different nutrient contents of stingless bee honey obtained from different botanical sources (64, 65).
Table 2.
Nutrient content of various samples of stingless bee honey.
| Sample | Scientific name | Ash (g/100g) | Moisture content (g/100g) | Carbohydrate (g/100g) | Protein (g/100g) | Ref. |
|---|---|---|---|---|---|---|
| Acacia | Acacia mangium | 0.17 | 31.00 | 68.33 | 0.50 | (63, 64) |
| Star fruit | Verrhoa carambola L | 0.20 | 31.00 | 68.60 | 0.20 | (63, 64) |
| Coconut | Cocos nucifera | 0.90 | 29.00 | 69.30 | 0.80 | (63) |
| Mangrove | Rhizophora mangle | 0.47 | 30.0 | 69.33 | 0.20 | (63) |
Ancient Egyptian surgeons used honey to treat wounds. Stingless bee honey can be used to treat inflammatory diseases (63, 65), as phenolic compounds present in it help reduce inflammation (63) by impeding the action of most prominent inflammatory regulators such as TNF-α, PGE2, and NO (63, 65). Pro-inflammatory cytokines such as IL-12 p40, IL-6 and TNF-α are engaged in the increase of inflammatory reactions and play a crucial role in immune response. IL-12 p40 plays a key role in immunoregulatory effects (66). IL-6 has spacious physio-cellular effects, such as regulating proliferation, differentiation, growth of cells, hematopoiesis, and inflammation (67). Monocytes and macrophages produce TNF-α which has involvement in immunoregulatory responses that are obliged to regulate immune homeostasis. On the other hand, overexpression of TNF-α is responsible for autoimmune diseases like RA and Crohn’s disease (68). PGE2 is a typical lipid mediators that produced from arachidonic acid by COX (69). In normal physiological situations, NO has anti-inflammatory property but overproduction of NO is responsible for inflammation in abnormal conditions (70). In addition, stingless bee honey works very well as an antioxidant by shutting down the activities of reactive oxygen species (63, 65) and acts as a good antimicrobial agent (63).
CAPSAICIN
Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is a major ingredient of hot peppers belonging to the genus Capsicum(71). Although, in general, it seems that capsaicin increases inflammation, studies have shown that sensitizing capsaicin also helps reduce inflammation (72). Recent studies have suggested that capsaicin may be used to reduce inflammation and joint pain. Usually, capsaicin reduces the efficiency of a pain transmitter called substance P (3). Capsaicin also inhibits lipopolysaccharide (LPS)-induced PGE2 production (71). When the COX2 protein level decreases, PGE2 release is inhibited (71). Interestingly, the COX2 protein level is not reduced by capsaicin; COX2 protein levels are decreased by capsazepine (a synthetic analog of capsaicin and antagonists of vanilloid receptor subtype-1 (71). Capsaicin and capsazepine accelerate each other’s actions, and both may affect disorders symptoms and cancer (71).
Recent studies have shown that capsaicin is also a potent gastroprotective agent (73), probably by enhancing gastric acid secretion. However, capsaicin has no role in acid secretion in GI applications, serosal application, or perfusion (73). It has been found that very low doses of capsaicin suppress acid secretion in pylorus-ligated rats (73). Furthermore, capsaicin may also be used in diabetes, obesity, itch, gastric, and urological disorders (72).
NATURAL MONOTERPENES
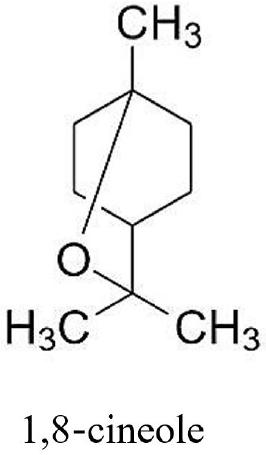
Monoterpenes have been known for centuries and can be derived from leaves, flowers, and plants as the main ingredient of essential oils (74). These essential oils can be obtained from gymnosperms, angiosperms, and in the plant families Santalaceae, Rutaceae, Lamiaceae, among others. A naturally occurring 1,8-cineole (eucalyptol) monoterpene (Fig. 2) is the major ingredient of many plant essential oils and is extracted from Eucalyptus globulus(75). This compound is well known for its spasmolytic and mucolytic actions on the respiratory system (75). It also has beneficial effects on inflammatory airway diseases such as chronic obstructive pulmonary disease (COPD) and asthma (74, 75). Recent clinical trials have demonstrated that long-term therapy with 1,8-cineole is very helpful in the control of asthma and COPD (75). This compound exerts its anti-inflammatory effect through two separate mechanisms: by reducing arachidonic acid (AA) metabolism of the COX and LOX pathways of PGE2 and LTB4, and by reducing the production of IL-1β (75, 76).
Apart from its anti-inflammatory activity, 1,8-cineole also acts as an antioxidant by inhibiting the action of ROS in COPD (75). Recent study showed that 1,8-cineole as a Th1/Th2 cytokine immunomodulator by its anti-inflammatory and antioxidant activities. However, the cause of mucus production was controlled by 1, 8-cineole as its multifunctional mechanisms (76). Various pollutants, including viruses and bacteria, can be inhaled, causing mucus hypersecretion and airway inflammation, possibly leading to acute respiratory distress and bronchitis (75). In this case, due to the bifunctional (antioxidant and anti-inflammatory) efficiency profile of 1,8-cineole, it can be used to treat bronchial complaints (75).
KAEMPFEROL
Kaempferol (3,4,5,7-tetrahydroxy flavone), a natural type of flavonoid, is characterized by a polyphenolic structure with a low molecular weight (MW 286.2 g/mol). It is abundant in fruits and vegetables, possesses many therapeutic effects such as anti-inflammatory, anticancer, antioxidant, antimicrobial, cardioprotective, and anti-diabetic (77, 78, 79). Kaempferol is found in much greater quantities in Angiospermae, Pinophyta, and Pteridophyta (79). The common plant sources of kaempferol-like flavonoids are Rosmarinus officinalis, Toona sinensis, Hypericum perforatum, Coccinia grandis, Sumbucus nigra, Glycine max, Aloe vera, Euphorbia pekinensin ilex, among other (77, 78, 79). The fractionation and screening of these plants produced kaempferol and its derivatives (77).
Kaempferol exerts its anti-inflammatory action through several mechanisms (79), including interference in the NF-κB-binding capacity of DNA, inhibiting the activation of pro-inflammatory mediators and enzymes (77, 78, 79). At the body’s physical and chemical trauma sites, kaempferol significantly inhibits the cyclooxygenase pathway, preventing the inflammatory process (77, 79). In vitro studies have also shown that kaempferol hinders the release of TNF-α, IL-18, IL-6, and IL-1β (77). It also exhibits inhibitory activity on activator protein-1, a transcriptional regulator of the inflammatory process (78, 79), and also inhibits Toll-like receptor 4 activity (77). Altogether, kaempferol might reduce inflammation through all of the above mechanisms.
VITAMINS AND MINERALS
Various studies have shown that certain vitamins and minerals have anti-inflammatory effects on IBD, arthritis, and periodontitis (80). The top anti-inflammatory vitamins are vitamins A, B, C, D, E, and K (81). Vitamin A is vital for maintaining the immune system (both innate and adaptive), and its supplementation is very effective against some major inflammatory diseases (82). Vitamin A has also been used as an anti-inflammatory agent for IBD, bronchial disorders, acne vulgaris, and some cancer states (82). Vitamin A exerts its anti-inflammatory action by suppressing the production of pro-inflammatory cytokines (83). Study showed that vitamin A has inhibitory effect on production of cytokines IL-1ra and IL-10 secretion (84). Supplementation with vitamin B (mainly B1, B2, B3, B5, B6, and B12) complex also exerts anti-inflammatory effects (85). It reduces the activity of inflammatory mediators such as IL-4, IL-10, TNF-α, and iNOS (85). Various studies have shown that dietary intake of vitamin C helps reduce inflammation (86). It improves endothelial vasoreactivity and hence acts as an anti-inflammatory agent (86). Vitamin D (especially vitamin D3) is very effective in treating inflammatory disorders such as arthritis, kidney diseases, and cancer (87). Vitamin D suppresses the action of the NF-κB signaling pathway and blocks the action of T helper 17 cells. It also suppresses the secretion of IL-12 and down regulates the activity of CD40 and CD86 (87). The vitamin E family of molecules contains four types of tocopherols that contribute significantly to the treatment of inflammatory disorders (88). Some studies have shown that vitamin K also has anti-inflammatory effects (89), and it is especially effective in treating rheumatoid arthritis. It reduces the expression of the NF-κB signaling pathway and decreases the activity of certain proteolytic enzymes, such as matrix metalloproteinases (89).
In addition to vitamins, some minerals such as calcium and magnesium can also act as anti-inflammatory agents (81). Currently, calcium and its salts (e.g., calcium citrate) are being used to treat a variety of inflammatory diseases (90). They suppress the expression of COX2, iNOS, and the NF-κB signaling pathways (90). Magnesium has also been found to be beneficial in reducing inflammation (91). It exhibits anti-inflammatory action by inhibiting the action of pro-inflammatory mediators (91)
CONCLUSION
Nature is an infinite resource for any medicine. In many cases, natural medicine can provide a much better solution than synthetic medicines. Due to the relatively lower incidence of side effects, the use of natural anti-inflammatory agents and supplements may be preferred. Natural anti-inflammatory drugs reduce body pain and inflammation very well. Numerous natural anti-inflammatory agents and supplements exist. To discover new anti-inflammatory agents, natural sources should be considered first. Further, the natural anti-inflammatory substances discussed in this review should attract the attention of researchers and pharmaceutical companies, to be validated in human clinical studies.