INTRODUCTION
The skin, the largest organ in the human body, acts as the main barrier between the body and the environment (1, 2). The skin is home to a diverse and complex microbial ecosystem, collectively known as skin microbiome (3). This ecosystem comprises a variety of commensal bacteria, fungi, viruses, and archaea that coexist in a homeostatic equilibrium (4). Skin microbiome composition varies across different body parts and is influenced by moisture, sebum content, and pH (5, 6). This site-specific diversity in skin microbiome composition is essential to maintain skin homeostasis and perform various functions, including protection against pathogens, immune modulation, and nutrient extraction (7).
Disruptions in the delicate skin microbiome balance, known as dysbiosis, have been linked to various skin disorders, including atopic dermatitis (AD), acne, psoriasis, and rosacea. These imbalances are often characterized by decreased microbial diversity and an overgrowth of specific pathogen species (8). In addition, emerging evidence suggests that alterations in skin microbiome may contribute to developing systemic diseases, such as diabetes, obesity, and inflammatory bowel disease (IBD) through the gut-skin axis (9).
This review provides a comprehensive overview of skin microbiome and examines its role in human health and dermatological and systemic diseases. It explores therapeutic strategies for microbiome modulation, including probiotics, prebiotics, antimicrobials, and lifestyle interventions. The review highlights challenges and limitations in skin microbiome research and discusses potential future applications of microbiome-based interventions in personalized medicine.
SKIN MICROBIOME: AN OVERVIEW
Composition and Diversity
Skin microbiome is a diverse community of various microorganisms, including bacteria, archaea, fungi, and viruses. The four predominant bacterial phyla are Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetes. Actinobacteria such as Cutibacterium thrive in sebaceous regions due to their lipid metabolism ability. Firmicutes, including Staphylococcus species, are commonly found in moist and dry environments. Proteobacteria and Bacteroidetes are distributed across various skin sites but vary considerably depending on the environment (10, 11, 12). Microbial composition differs significantly across body sites. Sebaceous areas, such as the face and back, contain high levels of Cutibacterium, whereas moist regions, such as the armpits and groin, harbor more Staphylococcus and Corynebacterium species. Dry areas, such as the forearms and legs, contain a diverse mix of Actinobacteria, Proteobacteria, and Bacteroidetes (13, 14). Environmental factors such as temperature, humidity, and sunlight, and host factors, including genetics, age, and immunity, significantly impact the composition and diversity of skin microbiome (10, 11, 12).
Factors Influencing skin microbiome
Skin microbiome evolves from a simple composition at birth to a complex composition in adulthood. Infants are colonized by maternal and environmental microbes, which stabilize over time (14, 15). Hormonal differences lead to skin microbiome variations between sexes. Men often have higher Cutibacterium levels due to increased sebum production. In contrast, women may have higher Staphylococcus and Corynebacterium levels (15). Different body sites provide distinct habitats, with sebaceous, moist, and dry areas, each supporting unique microbial populations (13, 14). Hygiene practices, such as washing and antimicrobial product use, can affect skin microbiome significantly. In addition, its excessive use can lead to dysbiosis and skin disorders (7). Climate, urban versus rural living, and pollutant exposure can alter microbial diversity and function. For example, urban environments may expose individuals to pollutants that affect skin health (7). Genetic factors and immune responses shape skin microbiome by influencing antimicrobial peptide production and other immune mechanisms (10, 12, 16).
Function of skin microbiome
Skin microbiome plays a crucial role in protecting the skin by outcompeting harmful pathogens for resources and space, thereby providing resistance to colonization. Commensal bacteria are beneficial microorganisms that inhabit the skin and produce antimicrobial substances that enhance the skin’s defense mechanisms against infections (10, 16). These substances maintain the balance between the skin’s immune system and the microbiome, prevent inflammation, and promote a healthy skin barrier. Commensal bacteria also stimulate the production of antimicrobial peptides, which help protect against infections. In addition, they promote regulatory T cell responses, which are essential for maintaining the immune balance and preventing autoimmune diseases (10, 16). Skin microbiome also plays a role in nutrient metabolism by breaking down complex molecules into simpler forms used by the hosts and the microbes. The microbiome regulates lipid production and other components essential for maintaining hydration and protecting against environmental insults, thereby maintaining the integrity of the skin barrier (17, 18).
SKIN MICROBIOME AND HUMAN HEALTH
Skin microbiome is essential for promoting skin health and overall well-being (Fig. 1).
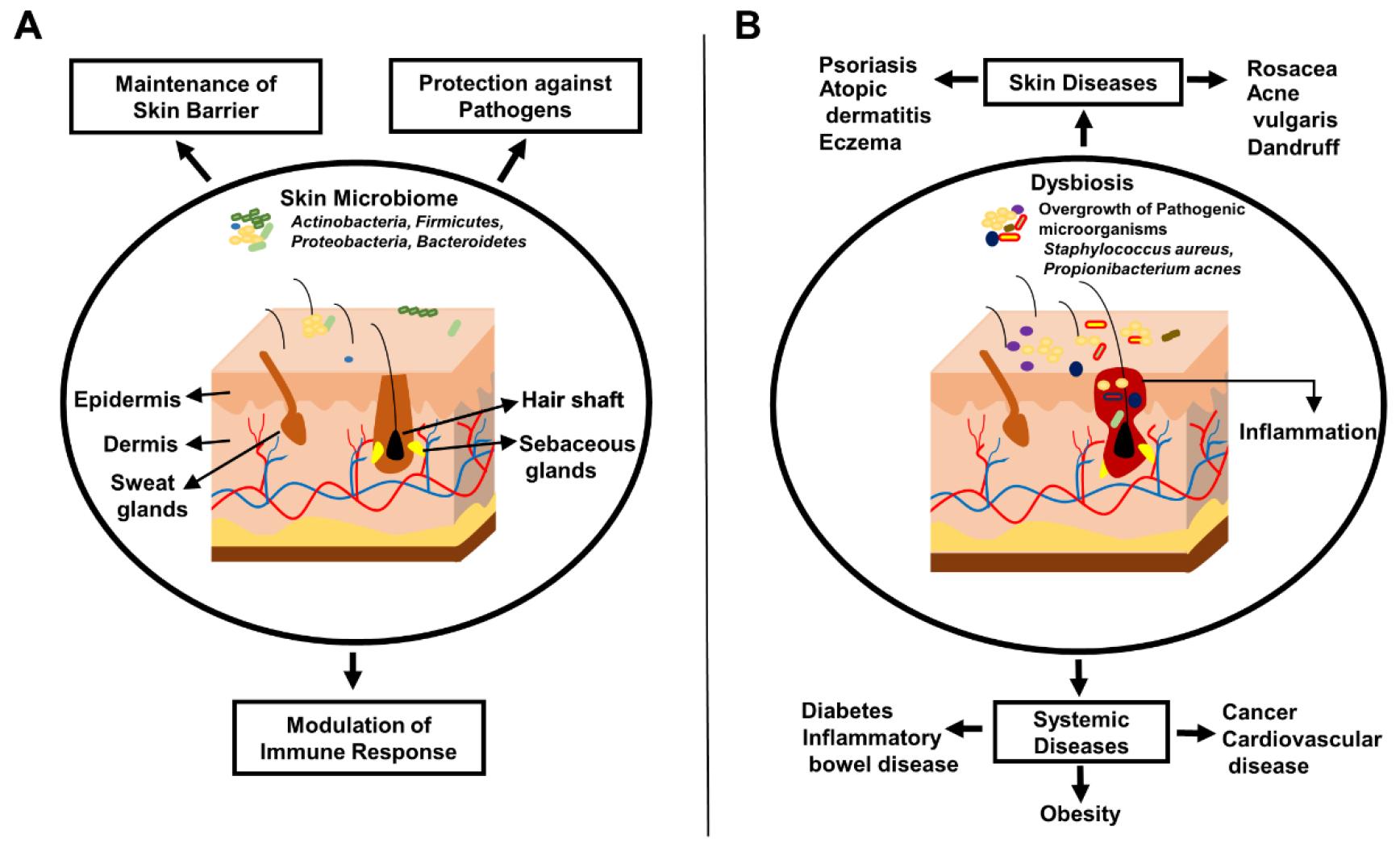
Fig. 1
Impact of skin microbiome on healthy and diseased skin. (A) Composition and protective roles of skin microbiome in healthy skin, including protection against pathogens, barrier maintenance, and immune modulation (B) Disruption of skin microbiome in various skin and systemic diseases, illustrating the links to altered microbial communities and their impact on skin pathology.
Role in Maintaining Skin Health
Skin microbiome is a valuable source of antimicrobial peptides (AMPs) and plays a crucial role in the skin’s innate immune defense. Commensal bacteria, including Staphylococcus epidermidis, produce AMPs that protect the skin from pathogenic microorganisms (19). AMPs can directly kill or inhibit the growth of harmful bacteria, fungi, and viruses, contributing to the skin’s protective barrier (19, 20).
Commensal microbes occupy skin niches, preventing harmful microbe colonization and overgrowth. Resource and space competition maintains microbial balance and protects against skin infections (21). Resident bacteria protect biological barriers against potentially harmful germs (22).
Skin microbiome is crucial for regulating innate and adaptive immune responses, contributing to immune tolerance towards harmless microbes while allowing effective responses against pathogens (19, 20). Early colonization of the skin by microbes is vital for developing a newborn’ immune system and preparing the skin for a life with many external influences (23).
Certain skin bacteria produce essential vitamins like vitamin K, promoting skin health. The microbiome breaks down sebum and other skin components, potentially enhancing nutrient accessibility for the host (20, 24).
Contribution to Overall Human Health
Skin microbiome impacts overall health through systemic immune modulation. It functions as a key interface between body and environment, influencing immune responses throughout the body (19, 20).
Skin microbes metabolize compounds like hormones and xenobiotics, potentially affecting systemic metabolism. This metabolic activity may significantly impact human health (20, 24).
The gut-skin axis concept highlights crosstalk between gut microbiome and skin. Evidence indicates mutual influence between gut and skin health, demonstrating interconnected microbial communities across the body (20, 22).
Factors Influencing a Healthy Skin Microbiome
Diet and nutrition play significant roles in maintaining a healthy skin microbiome. A balanced diet rich in prebiotics can promote the growth of healthy bacteria on the skin (25). Consuming fiber-rich foods and avoiding excessive sugar intake supports a balanced microbiome (25). Ultraviolet (UVR) radiation, pollution, and stress can alter microbial communities on the skin. Managing these exposures and adopting a healthy lifestyle can help maintain a balanced skin microbiome (20). Proper hygiene is vital, but over-cleansing or harsh products can disrupt skin microbial balance. Using gentle, microbiome-friendly products and limiting antimicrobial agents preserves a healthy skin microbiome (26, 27). Regular cleaning of personal items, such as phones and keyboards, frequent washing of bed linen, and avoiding touching the face can also contribute to maintaining a healthy skin microbiome (7).
Skin Microbiome and Melanin Production
The relationship between skin microbiome and melanin production is an emerging area of research. Direct evidence is limited; however, some studies have suggested that certain skin microbes influence melanogenesis. For example, Propionibacterium acnes, a common skin commensal, produces porphyrins that affect melanin production (28). In addition, S. epidermidis produces short-chain fatty acids through fermentation, which may also modulate skin pigmentation by influencing melanin production pathways (29). Notably, some species of Malassezia, a common fungus on human skin, cause conditions such as pityriasis versicolor, characterized by hyper- or hypopigmented skin patches. Malassezia species also produce melanin, which protects the skin and microorganisms from UVR damage (30). In addition, Malassezia furfur produces pityriacitrin, an ultraviolet-absorbing compound that protects fungal colonies from UVR. Escherichia and Enterococcus species produce serotonin, a neurotransmitter involved in skin pigmentation. Serotonin is implicated in various skin functions, including pigmentation, suggesting that these serotonin-producing bacteria influence skin color (30).
SKIN MICROBIOME AND DISEASES
Skin Microbiome dysbiosis is implicated in various skin and systemic diseases (Fig. 1) (31).
Atopic Dermatitis
AD is a chronic inflammatory skin condition characterized by itchy, red, and swollen skin (32). Individuals with AD exhibit deficiency in AMPs, such as cathelicidins and defensins, which are crucial for maintaining skin microbiome balance and protecting against pathogenic bacteria (19). A hallmark of AD is an overgrowth of Staphylococcus aureus on the skin. This bacterium can exacerbate inflammation and skin barrier dysfunction, contributing to disease severity (33). Patients with AD often exhibit reduced microbial diversity and altered microbial composition, significantly decreasing beneficial bacteria such as S. epidermidis(16).
Acne Vulgaris
Acne vulgaris is a common skin disorder, particularly among adolescents, characterized by pimples, blackheads, and cysts (34). It is associated with an increased population of Propionibacterium acnes and other anaerobic bacteria in the pilosebaceous unit of the skin (35). These bacteria contribute to inflammation and excessive sebum production, leading to the formation of acne lesions (36).
Psoriasis
Psoriasis is a chronic autoimmune skin disease characterized by scaly red patches on the skin (37). Studies have shown that individuals with psoriasis have altered diversity and abundant specific microbial taxa compared with healthy controls. In healthy people, Kocuria and Cutibacterium were more abundant, whereas Staphylococcus was more prevalent in people with psoriatic disease (38). The dysbiotic skin microbiome in psoriasis may contribute to disease pathogenesis by interacting with the immune system and skin barrier function (39).
Other Skin Conditions
Dandruff, eczema, and rosacea are additional skin conditions implicated in skin microbiome dysbiosis.
Dandruff
Malassezia species play a significant role in dandruff, and scalp conditions are associated with shifts in their abundance (40).
Eczema
Alterations in skin microbiome, including decreased diversity and shifts in microbial composition, have been observed in individuals with eczema. Approximately 90% of persons with eczema show S. aureus colonization and decreased microbial diversity (41). In addition, research has revealed clear distinctions between the baseline skin microbiomes of persons prone to eczema and healthy individuals. These differences include microbiome signatures enriched in possible pathobionts of Streptococcus, Gemella, and Haemophilus (41).
Rosacea
Dysbiosis in rosacea involves an increased abundance of certain bacteria and altered microbial diversity, contributing to chronic inflammation and vascular dysfunction in the skin. Research has identified several microorganisms, including Bacillus oleronius, Cutibacterium acnes, S. epidermidis, and Demodex folliculorum, as possible causes of rosacea (42). In untreated patients with rosacea, the predominant bacterial genera were Staphylococcus, Cutibacterium, Pseudomonas, Corynebacterium, Acinetobacter, and Snodgrasella. The two most common bacteria on the skin of patients with rosacea were S. epidermidis and Keratomycesacnes. S. epidermidis was the most prevalent bacterial species when examined at the species level, followed by Corynebacterium tuberculostearicum, Corynebacterium acne, and Stenotrophomonas rootophilus(42).
Systemic Diseases Associated with Skin Microbiome Alterations
Diabetes
Diabetes mellitus is a metabolic disorder characterized by high blood sugar levels. Individuals with diabetes show altered composition and diversity of their skin microbiome, which may influence the disease progression and complications. A study examined the microbiome composition of 120 hospitalized patients with diabetic foot ulcers (DFUs) and discovered that 81.66% had an infection, with 23.3% being polymicrobial infections. The most frequent colonizers were S. aureus, Escherichia coli, and Pseudomonas aeruginosa (40.81%, 34.69%, and 30.61%, respectively) (43). Using 16S ribosomal deoxyribonucleic acid amplicon sequencing, another study examined the DFUs microbiome, and the most frequently isolated organism from 52 samples was Staphylococcus species, indicating that these species are especially harmful to DFUs. Phylogenetic analysis revealed that S. aureus and S.epidermidis accounted for 96.5% and 0.4%, respectively (43). Dysbiosis of skin microbiome in patients with diabetes may exacerbate skin infections and impair wound healing, contributing to diabetic complications (44).
Obesity
Obesity is a complex metabolic disorder associated with the accumulation of excessive body fat. Individuals with obesity have altered skin microbiomes. Reportedly, changes in the composition of skin microbiome are linked to varying body mass index levels, and skin microbial diversity is lower in persons who are overweight and obese (45).
Inflammatory bowel disease
IBD includes Crohn’s disease and ulcerative colitis, characterized by chronic inflammation of the gastrointestinal tract (46). The gut-skin axis plays a crucial role in IBD, with dysbiosis of the gut microbiome potentially influencing skin microbiome and contributing to skin manifestations (8, 47). Skin manifestations in patients with IBD, such as erythema nodosum and pyoderma gangrenosum, may be linked to skin microbiome dysbiosis and systemic inflammation (48).
Other Systemic Conditions
Cardiovascular disease
Skin microbiome dysbiosis is linked to several chronic inflammatory dermatological conditions, including psoriasis and AD, which are known risk factors for cardiovascular diseases (49). In addition, dysbiosis in skin microbiome composition may affect endothelial dysfunction and systemic inflammation, contributing to cardiovascular disease development and progression (49).
Cancer
The role of skin microbiome in cancer pathogenesis is an active area of investigation with potential implications for immune surveillance and tumor development, especially in non-melanoma skin cancers such as squamous cell carcinomas (50, 51). Skin microbiome dysbiosis can disrupt redox homeostasis, leading to oxidative stress and possibly influencing skin cancer development (52). Research on the dysbiosis in skin microbiome during melanoma progression revealed that in the microbiome of healthy skin, Lactobacillus, Clostridium sensu stricto cluster 1, and Corynebacterium were the most abundant genera, whereas Fusobacterium, Trueperella, Staphylococcus, Streptococcus, and Bacteroides were more abundant in melanoma tissue. The fecal microbiota of MeLiM piglets (a porcine model) has been linked to Bacteroides, Fusobacterium, and Escherichia coli (53). Researchers also employed swab samples and 16S ribosomal ribonucleic acid gene sequencing in a tumor biopsy study and discovered that S. aureus grew more quickly on the skin of patients with squamous cell carcinoma than in healthy individuals (54). In addition, they observed a strong correlation between an increase in S. aureus and skin keratosis, which are precancerous lesions associated with squamous cell carcinoma (54). Malignant melanoma, the most dangerous type of skin tumor, is responsible for approximately 75% of deaths from skin cancer (54). Compared with normal skin, skin microbiome in animal melanoma models shows variations in the importance of microbial composition and diversity. Skin samples from patients with melanoma showed increased Fusobacterium and Eucoccus (Trueperella) (54).
Skin Microbiome and Immunodeficiency
Immunodeficiency alters skin microbiome dynamics, thereby affecting disease susceptibility and management. Individuals with immunodeficiency disorders exhibit distinct alterations in skin microbiome, which may exacerbate skin infections and dermatological complications (55, 56). The skin microbiota of mice with long-term immunodeficiency changes has Staphylococcus predominating without being detrimental. This emphasizes the intricate host-commensal interaction in the skin (57). Skin microbiome dysbiosis can also induce immunodeficiency-like cutaneous bacteria such as S. aureus, increase myeloid-derived suppressor cell growth, prevent antimicrobial responses, and exacerbate inflammation, all of which contribute to immunodeficiency and systemic immune suppression (58).
THERAPEUTIC MODULATION OF SKIN MICROBIOME
The therapeutic modulation of skin microbiome is a promising strategy for treating various skin disorders and enhancing skin health (Fig. 2).
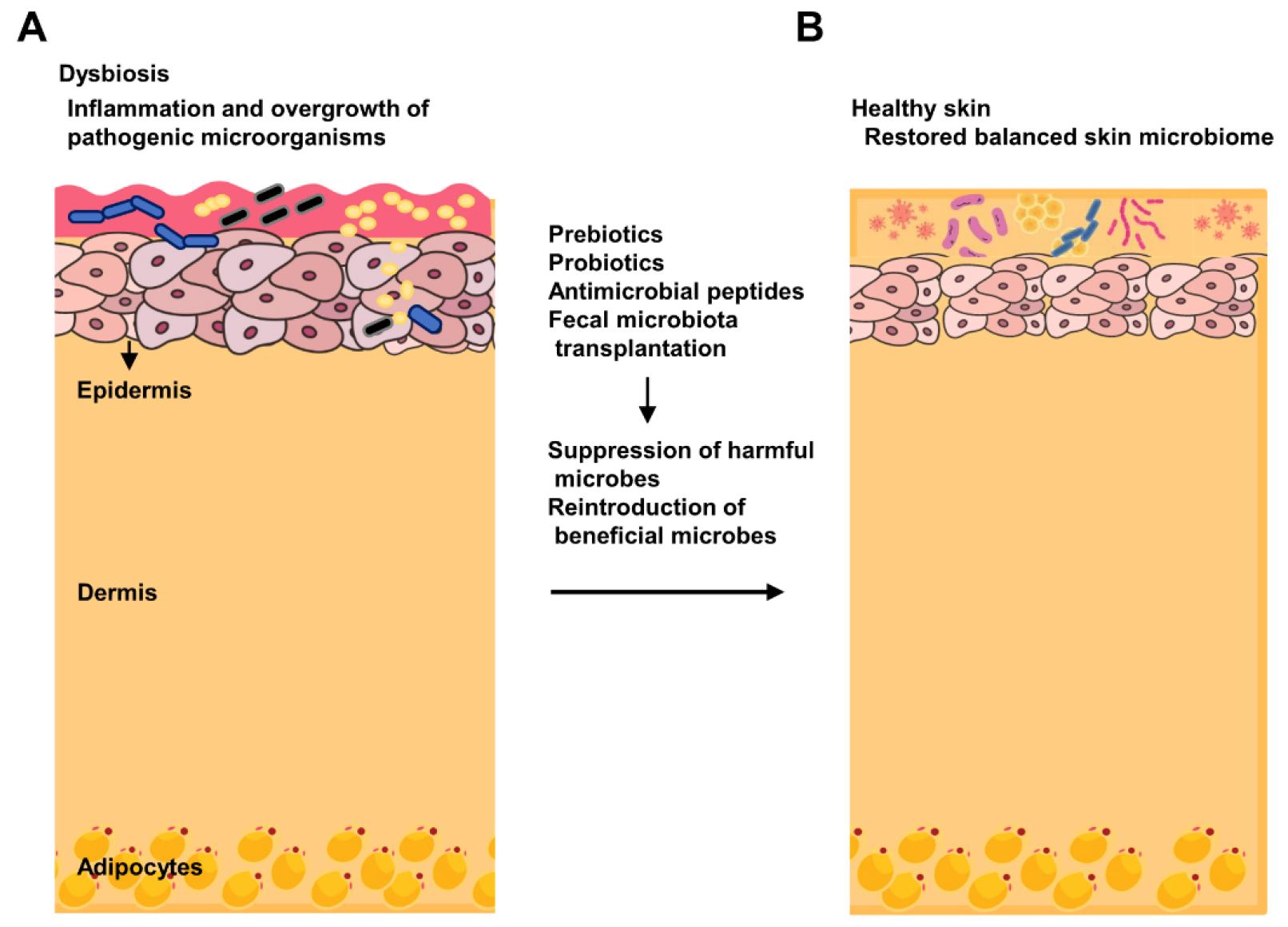
Fig. 2
Therapeutic modulation of skin microbiome. (A) Dysbiosis in skin microbiome, characterized by an overgrowth of harmful microbes and a deficiency of beneficial microbes (B) The restoration of microbial balance through treatments such as probiotics, prebiotics, antimicrobial peptides, and fecal microbiota transplantation, demonstrating the suppression of harmful microbes and reintroduction of beneficial microbes.
Probiotics and prebiotics offer promising therapeutic approaches for modulating skin microbiome and improving various dermatological conditions (20, 30). Probiotics are live microorganisms that confer health benefits, while prebiotics are non-digestible ingredients that promote the growth of beneficial microorganisms (20, 59). They modulate the immune system, inhibit pathogens, and restore healthy microbiome balance (20, 59).
In AD, oral and topical probiotics have shown efficacy in reducing symptoms by enhancing skin barrier function and reducing inflammation (60). For psoriasis, synbiotics combined with topical treatments have demonstrated improvements in severity indexes (59). Probiotic solutions derived from healthy skin microbiota have effectively treated acne by reducing Propionibacterium acnes colonization (30).
Preliminary studies have suggested that oral and topical prebiotics, probiotics, and postbiotics may improve signs of skin aging by enhancing skin microbiome diversity (61). Thermal spring water, containing probiotics and prebiotics, has shown promise in this regard (61). Further research is required to understand fully the mechanisms involved in establishing standardized treatments and the long-term implications (20, 59, 61).
Fecal microbiota transplantation (FMT) is a potential therapeutic approach to modulate skin microbiome, particularly in AD and other skin conditions. FMT aims to restore gut microbiota homeostasis, which influences skin microbiome through the gut-skin axis (62). Studies in mouse models have shown that FMT affects the recovery of AD-related skin lesions and inflammation. FMT treatment leads to a faster reversal of epidermal thickening and suppression of inflammatory cytokines associated with AD (63). FMT has shown a beneficial impact on the gut microbial composition in mice with AD, increasing the ratio of Firmicutes to Bacteroidetes and the abundance of butyrate-producing bacteria (63).
FMT can restore the Th1/Th2 balance, modulate regulatory T-cells, and reduce Immunoglobin E levels and the numbers of mast cells, eosinophils, and basophils, all of which are important in the pathogenesis of AD (62, 63). FMT has also shown potential for treating conditions like alopecia universalis and psoriasis. However, further research is needed (64).
Human clinical trials are required to fully evaluate the safety and long-term effects of FMT on skin microbiome modulation under various dermatological conditions.
Another strategy is the use of antibacterial peptides. Antimicrobial peptides are synthesized or naturally occurring small proteins with broad-spectrum antimicrobial activities (65). They are interesting options for topical therapies against various skin infections and inflammatory disorders because they disrupt bacterial membranes or hinder vital cellular processes (66).
CONCLUSION
Skin microbiome is essential for maintaining skin health and overall human well-being. Recent studies have shown intricate interactions between skin microbiome, the immune system, and environmental factors. Skin microbiome imbalance is linked to various skin disorders and systemic diseases, making it a potential therapeutic target.
Characterizing and understanding the highly diverse and dynamic skin microbiome is challenging. Significant variations in microbial communities exist between individuals and across different body sites, complicating the establishment of a “normal” skin microbiome. Determining whether microbial changes cause or result from skin conditions remains difficult. Long-term studies integrating genomics, transcriptomics, and metabolomics provide a more comprehensive understanding of skin microbiome dynamics.
Further research into microbiome-host interactions and animal models will clarify the functional roles of specific microbes. Rigorous clinical trials are needed to evaluate the effectiveness of microbiome-based therapies for skin disorders. Understanding individual skin microbiome profiles may lead to personalized treatments. Insights into skin microbiome may inform public health policies and preventive measures to maintain skin health.




