INTRODUCTION
The Bacillus cereus is a gram-positive, aerobic, rod-shaped microorganism (1), has the remarkable ability to endure dry and hot conditions, and can remain dormant for several years as a spore (2). It thrives optimally at 30°C but can also grow within the range of 10°C to 49°C and at pH levels ranging from 4.9 to 9.3 (3). Among Bacillus spp., B. cereus is one of the most common bacteria on Earth (4). Its natural habitat includes decaying organic materials, soil, freshwater, seawater, vegetables, and the intestinal tract of non-vertebrate organisms (5). Additionally, it is frequently found in various foodstuffs such as cereals (rice, wheat, and derivatives), dairy products, dry foods, eggs, spices, meats, and vegetables (6).
Bacillus and related genera have garnered attention from food producers due to their resilient endospores (7). The colonization of B. cereus in the human intestine is also feasible due to its ubiquitous presence (8). Reports by Schmitt et al. (1976) (9) have demonstrated evidence of food poisoning resulting from the consumption of powdered milk products contaminated with B. cereus. Food poisoning strains of B. cereus produce four different enterotoxins, including two enterotoxic proteins and two proteinous complexes such as nonhemolytic enterotoxin and hemolysin BL (HBL) (10). B. cereus causes two types of foodborne diseases: emetic intoxication (vomiting) due to ingesting cereulide toxin produced in foods and diarrheal infection caused by ingestion of bacterial cells/spores capable of producing enterotoxin in the small intestine (11). Food poisoning incidents caused by B. cereus have been reported across Europe (12, 13, 14, 15, 16), Latin America (17), and Australia (18). Non-gastrointestinal diseases such as endocarditis and endophthalmitis have also been reported due to B. cereus(19).
Heat-resistant spores and vegetative forms of B. cereus are common contaminants in various food types, including milk and dairy products (20), making them easily spreadable. Consequently, processed, pasteurized, and other heat-treated food products are at risk of contamination (21). Powdered infant formula (PIF) and powdered follow-up formula (PFUF), marketed as breast milk substitutes for infants, may serve as the sole source of nutrition for some infants (22). Neonates and young children, reliant on powdered infant formula and cereals along with breast milk, are frequently exposed to B. cereus spores and vegetative forms, prevalent in cereals or their derivatives (23, 24), ready-to-serve foods (25), pulses, and rice (26). As neonates and young children solely dependent on powdered infant formula and different cereals along with breast milk, they often suffer from foodborne pathogens that may lead to higher mortality due to their underdeveloped immune and metabolic systems (27). Additionally, antibiotic treatment remains the primary method for treating bacterial infections, including those caused by B. cereus. However, the extensive use of antimicrobials has led to the emergence of antibiotic-resistant strains, resulting in routine treatment failures (28). Antibiotic resistance can spread within the bacterial population through bacterial transfer among human populations, resistant gene transfer between two bacteria (often on plasmids), and resistant gene transfer between genetic elements of two bacteria (usually on transposons) (29). Antimicrobial-resistant genes present in foodstuffs as DNA fragments or carried by bacteria and bacteriophages may pose potential hazards to public health safety. The World Health Organization (WHO) has recently expressed concern about the preparation, hygienic handling, and supply of powdered infant formulas (PIFs) in healthcare settings (30).
Therefore, the microbiological safety of PIF is of utmost importance. The incidence of foodborne illnesses has surged worldwide, presenting a significant issue in developing countries like Bangladesh (30). Hence, this study aims to examine the prevalence of B. cereus in consumed PIF and cereals, as well as assess the antibiotic sensitivity of B. cereus isolates.
MATERIALS AND METHODS
Sample Collection
Six powdered infant formulas (PIF-1, PIF-2, PIF-3, PIF-4, PIF-5, PIF-6) and four cereal products (Rice pudding, Suzi, Cerelac, Mixed pudding) (C1, C2, C3, C4 respectively) were collected directly from local markets in Bangladesh. During collection, the manufacturing and expiration dates of each product were checked. Only products with more than 50% of their shelf life remaining were selected for the experiment. The samples were then transported to the lab and stored at 4°C until further analysis.
Microbial load determination
The spread plate method was used to estimate the total viable plate count. A small volume of a diluted microbial suspension (typically 0.1 mL) was spread evenly across the surface of a nutrient agar plate using a sterile glass spreader or L-shaped rod. The plates were then incubated under appropriate conditions to allow microbial growth. The total viable number of discrete colonies from different plates of each sample was counted after 24 hours of incubation at 37°C. Plates with colonies between 30 and 300 were accepted for microbial load determination. If the count fell outside this range, the procedure was repeated with the next or previous dilution to obtain countable colonies. The microbial load was then determined using the following equation:
Number of CFU/g = Number of Colony/ (Volume plated in ml × total dilution used) (31).
Isolated colonies were counted as colony-formation units (CFU) per gram.
Isolation of bacterial strain
One gram of collected samples was diluted with 9 ml of sterilized distilled water and appropriately diluted suspension (0.1 mL) was spread-plated on B. cereus selective agar (BCSA) base and incubated at 35°C for 24 - 48 h. Characteristic turquoise to peacock blue colonies surrounded by a zone of precipitate of the same color were regarded as presumptive B. cereus. Distinct colonies were purified and maintained on nutrient agar (per liter: 5g peptic digest of animal tissue, 3g beef extract, and 15g agar, pH 7) slants at 4°C (32).
Biochemical test for taxonomic identification
A total of 28 isolates were isolated from PIF (21 isolates) and Baby food samples (7 isolates) were subjected to several biochemical tests for taxonomic identification which included MIU (Motility, Indole, and Urease Test), casein test, starch hydrolysis test, citrate utilization test, Mannitol fermentation test, etc. Gram staining was also done. Confirmation of B. cereus was done by endospore staining. As positive control, Bacillus cereus ATCC 11778 was used and E. coli ATCC 25922 was used in this experiment as negative control.
PCR amplification and 16S rRNA gene sequencing
Selected isolates were subjected to PCR amplification of partial sequence of the 16S rRNA gene of the bacterium for identification. A commercial DNA extraction kit (Promega, USA) was used according to the supplier’s instructions for DNA isolation. The partial sequence of the 16S rRNA gene was amplified with universal primer 1492R (5′-TACGGCTACCTTGT TACGACTT-3′) and 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) in the present investigation. DNA amplification was carried out in a thermal cycler (Applied Biosystems 2720) using the following conditions: 35 cycles, preheating 95°C for 5 minutes, denaturation at 95°C for 30 seconds, annealing at 57°C for 30 seconds and extension at 72°C for 2 mins. Amplification products were analyzed by gel electrophoresis in 1% agarose gel with buffer solution (TBE buffer). Then PCR products were stained with 0.1% ethidium bromide and illuminated under UV light.
The PCR products of ~1470bp were then sequenced by commercial service providers. The sequences were analyzed and the identification of bacterial isolates was accomplished by comparing the sequences with sequences available in public databases of NCBI Blast (Basic Local Alignment Search Tool) using the BLAST search engine (http://blast.ncbi.nlm.nih. gov/Blast.cgi). MEGA version 6 was used for the multiple sequence alignment and phylogenetic tree (33)). The phylogenetic trees of the selected three isolates were produced using currently obtained sequence data and retrieving sequences from the NCBI database.
Susceptibility of bacterial isolates to different antibiotics
The sensitivity of B. cereus to 15 antibiotics namely (Kanamycin, Amoxycilin, Aztreonam, Penicillin-G, Cefixime, Gentamycin, Cefotaxime, Cefepime, Tigecycline, Ceftriaxone, Ciprofloxacin, Meropenem, Cotrimoxazole, Amikacin, Ceftazidime) was studied by using Standard Kirby-Bauer disk diffusion method (34). These 15 antibiotics were chosen due to their wide spectrum activity against both Gram-positive and Gram-negative bacteria. The B. cereus isolates from preserved slants were inoculated into a tube of NA broth medium and allowed to grow at a specified density at 37°C for 24 hours. A sterile swab is dipped in the liquid culture. The swab is streaked evenly over a plate of sterile Mueller-Hinton agar plate. After the inoculum was dried, the antimicrobial disks were put to the surface of the plates with sterile forceps. The Mueller-Hinton agar plates were incubated for 24 h at 35 ± 2°C, and the inhibition zone was measured. All results were recorded appropriately and interpreted using the National Committee for Clinical Laboratory Standards interpretation chart (35). The isolates were classified as susceptible (S), intermediate (I), or resistant (R) according to CLSI and interpreted according to the zone diameter interpretation criteria for B. cereus.
Calculation of multi-drug resistance index
The multi-drug resistance (MDRI) index for each bacterial isolate against the antibiotics tested was calculated using a formula. Multi-drug resistance index is calculated by the ratio of the number of resistant antibiotics to which isolates are resistant to the total number of antibiotics to which the organism is tested.
MDR Index value =a/b
Where, a=Number of antibiotics to which the bacteria is resistant
b=total number of antibiotics tested to bacteria
RESULTS
Bacterial load of the samples
In our experiments, the average CFU for individual powdered infant formula (PIF) samples and the overall average CFU for all PIF samples were significantly lower than the corresponding values for cereal samples. However, the Colony Forming Units (CFU) or viable plate counts for all PIF and cereal samples exceeded the acceptable limits as per the criteria set by the International Commission on Microbiological Specifications for Foods (ICMSF) (Table 1).
Table 1.
Bacterial load (CFU/g) of different samples
| Sample name | CFU (mean ± SD) | ICMSF accepted range of CFU* | ||
|---|---|---|---|---|
| m | M | |||
| Powdered Infant Formula (PIF) | PIF-1 | 28.9x103±1.9x103 | 103 | 104 |
| PIF -2 | 18.2x103±1.8x103 | |||
| PIF -3 | 46x103±4.8x103 | |||
| PIF -4 | 20.5x103±2.1x103 | |||
| PIF -5 | 16.6x103±480 | |||
| PIF -6 | 11.5x103±495 | |||
| Cereals | C-1 | 8.4 x108±9.3x106 | 105 | 106 |
| C-2 | 2 x1010±1.4x108 | |||
| C-3 | 8.3 x107±2.4x105 | |||
| C-4 | 2.2x1010±1.4x108 | |||
Morphological and biochemical characterization
Biochemical and morphological characterization of 28 isolates (21 from powdered infant formula [PIF] samples and 7 from baby food samples) was conducted. Among these, 12 isolates exhibited motility and a rod-shaped morphology. These 12 isolates were subjected to further biochemical tests, where they tested positive for gram staining, indicating they are Gram-positive bacteria. They also showed positive results for citrate utilization, which means they can utilize citrate as a sole carbon source, and endospore staining, indicating their ability to form endospores, a key characteristic of certain bacterial genera. Conversely, these 12 isolates tested negative for indole production, urease activity, and mannitol fermentation. The absence of indole production suggests they do not possess tryptophanase, the enzyme responsible for converting tryptophan to indole. The negative urease test indicates they do not hydrolyze urea to ammonia and carbon dioxide, and the negative mannitol test suggests they do not ferment mannitol. Additionally, these isolates were positive for casein and starch hydrolysis tests, demonstrating their ability to produce extracellular enzymes such as amylase and lipase. Amylase breaks down starch into simpler sugars, while lipase breaks down casein, a protein found in milk. Combining these morphological and biochemical characteristics, the 12 isolates were confirmed to be Bacillus cereus strains. B. cereus is known for its rod shape, motility, Gram-positive staining, ability to form endospores, and production of amylase and lipase. These results align with the known properties of B. cereus, confirming the identification of these isolates (Table 2).
Table 2.
Results of biochemical tests and staining of different isolates
Molecular characterization
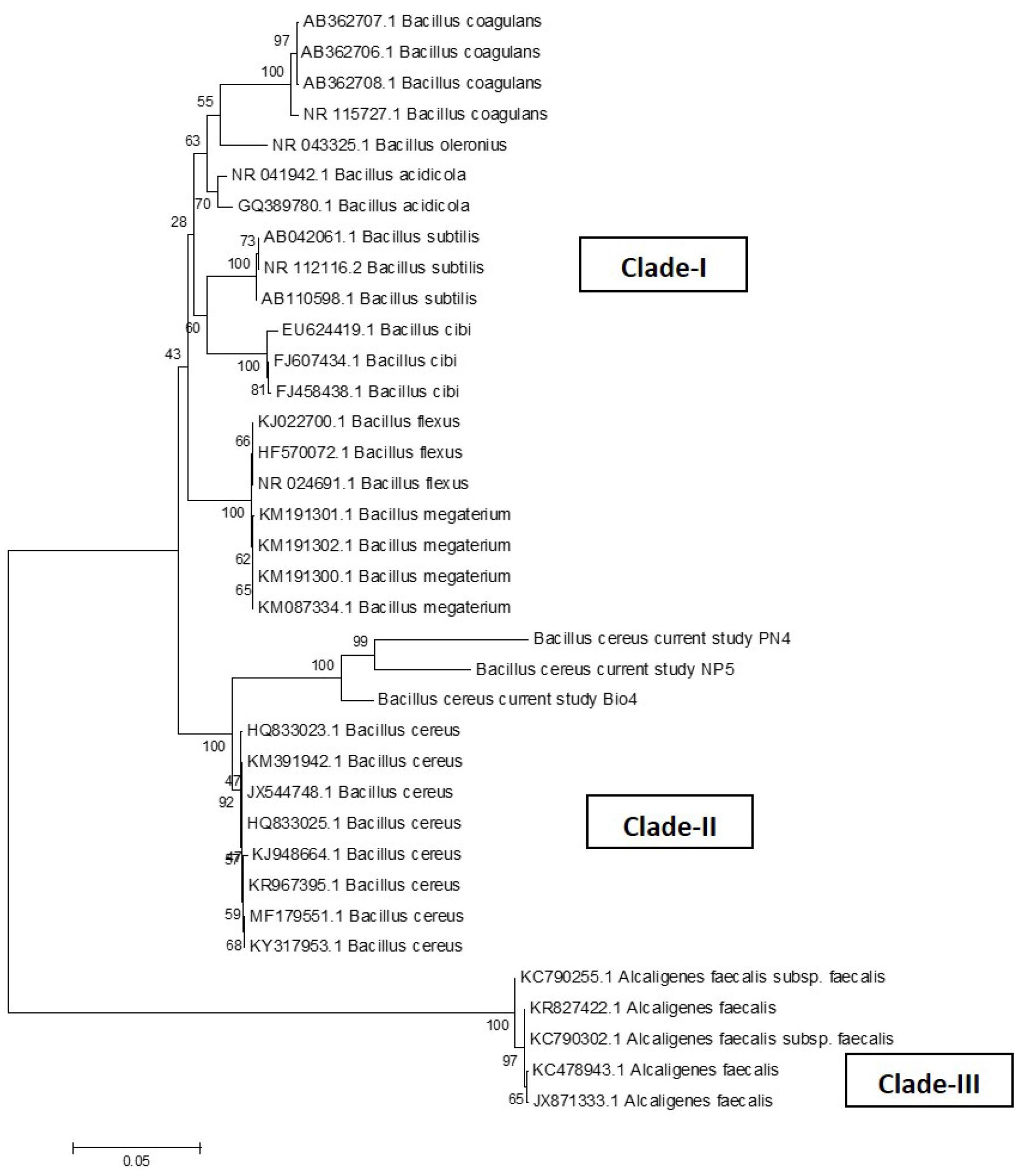
Three bacterial isolates out of twelve were isolated from enrichment cultures that were maintained at 30°C for 24 hours. Then DNA samples of the isolates were visualized after PCR which was ~1470 base pairs (Fig. 1). Purified PCR products were subjected to Sanger sequencing and DNA sequences (Bacillus cereus Bio4, Bacillus cereus PN4, and Bacillus cereus NP5) were matched with the NCBI database. Generated phylogenetic trees using currently studied three 16S rDNA gene sequences and 32 different Bacillus cereus strains and 5 different Alcaligenes faecalis strains from Gene Bank have been shown in Fig. 2. In the Neighbor-joining tree, there were three major clades found; clade-I had many bacterial species of Bacillus namely Bacillus coagulans, B. acidicola, Bacillus subtilis, Bacillus cidi, B. flexus, and Bacillus megterium. Clade-II illustrated the phylogenetic relationship among currently studied Bacillus cereus and other B. cereus. Our bacterial isolates exhibited 100% similarity with other described Bacillus cereus-HQ8333023.1; KM391942.1; JX544748.1; HQ833025.1; KJ948664.1; KR967395.1; MF179551.1; KY317953.1 in this clade. The third clade represents a separate clade in which several Alcaligenes faecalis were present.
Antibiotic sensitivity
In the antibiotic sensitivity assessment, all three bacterial isolates demonstrated sensitivity to the antibiotics kanamycin, gentamycin, amikacin, tigecycline, and ciprofloxacin. This indicates that these antibiotics were effective in inhibiting the growth of the isolates. Conversely, the isolates showed resistance to a range of other antibiotics, including amoxicillin, aztreonam, penicillin-G, cefixime, cefotaxime, cefepime, ceftriaxone, meropenem, cotrimoxazole, and ceftazidime. The resistance to these antibiotics suggests that the isolates possess mechanisms that render these drugs ineffective. The multi-drug resistance index (MDRI) value for the three isolates was calculated to be 0.67. Detailed results of the antibiotic sensitivity assessment are presented in Table 3.
Table 3.
Antibiotic Sensitivity pattern of Bacillus sp. isolates against different antibiotics and Multi-drug Resistant Index (MDRI)
DISCUSSION
In the present study, the colony-forming units (CFU) of powdered infant formula (PIF) ranged from 11.5x103 CFU/g to 46x103 CFU/g, while cereals ranged from 8.3 x107 CFU/g to 2.2x1010 CFU/g. These values exceeded the acceptable level recommended by the International Commission on Microbiological Specifications for Foods (ICMSF). Among the various bacterial isolates, the most abundant in both PIF and cereals, with 12 out of 24 isolates identified as B. cereus. This bacterium is frequently found in milk, dairy products, and products containing dried milk (20). European Food Safety Authority guidelines recommend that B. cereus spore levels in powdered infant formula be kept as low as possible (< 100 CFU/g) and be monitored closely by the manufacturer (36, 37). However, in our study, CFU values in all samples exceeded the standard acceptance limit suggested by ICMSF.
Previous studies have also documented the presence of Bacillus cereus in PIF and cereals. Kulshreshtha (38) reported the first outbreak of B. cereus food poisoning among children in India in 1978 due to the consumption of milk powder. Rahimi et al. (39) reported in 2013 the presence of B. cereus and its enterotoxigenic genes in 42% of infant food samples in Iran. Yu et al. (40) showed that ready-to-eat (RTE) foods caused food poisoning due to high levels of contamination. Similarly, Zhao et al. (41) demonstrated the prevalence of B. cereus isolated from powdered infant formula, raw milk, pasteurized milk, ultra-high-temperature milk, and cheese. These findings are consistent with our present results.
B. cereus can cause food poisoning even at very low concentrations; levels of about 103B. cereus g–1 is considered unsafe for human consumption (42). Since RTE foods are not commonly sterilized by heat treatment before consumption, the presence of B. cereus may lead to foodborne diseases after consumption. Bacterial contamination often occurs due to poor handling and the addition of raw ingredients during drying, processing, and packaging (43). Furthermore, the bacterial load may increase during the transportation, processing, and storage of milk and milk products. Processing equipment may serve as reservoirs for B. cereus milk recontamination, especially post-pasteurization contamination (44, 45). B. cereus is capable of forming viscous, highly heat and drought-resistant spores, which adhere to surfaces, particularly hydrophobic surfaces, making their removal from preparation surfaces on production lines challenging (46). Moreover, B. cereus spores are extremely heat resistant, so cooking does not destroy them. Impure water is also frequently used in the preparation of powdered infant formulas (47). Therefore, caution should be exercised in using water for the preparation of infant formula, and contamination-free safe water should be used.
In the current study, the presence of different enzymes was observed in bacterial isolates. All B. cereus isolates contained both amylase and lipase enzymes, which are heat-stable. Lipase, especially in processed milk and milk products, could be a concern as it can survive processing temperatures and contribute to spoilage even if vegetative cells are eliminated during processing. These lipase and amylase enzymes significantly reduce the shelf life of processed milk and milk products by degrading milk components and additives (48).
The minimum multi-drug resistance index (MDRI) value of Bacillus cereus isolates was 0.67, indicating a higher health risk, as it falls above the range of 0.36 to 0.57. An MDR index value of 0.67 also indicates that each isolate is resistant to 67% of the antibiotics tested. This high level of resistance is concerning, as it implies that these bacterial isolates could pose significant treatment challenges if they were to cause infections. The findings underscore the importance of ongoing surveillance and judicious use of antibiotics in both clinical and agricultural settings to mitigate the spread of resistant bacterial strains. The occurrence of multidrug resistance among foodborne bacterial pathogens can be a significant health concern for human babies (49). According to the results of our antimicrobial sensitivity testing, suspected B. cereus infections should not be clinically treated with broad-spectrum cephalosporins and penicillin. This antibiotic resistance may be attributed to the frequent use of these antibiotics in treating cow infections, increasing milk yield, and preventing contamination by adding them in bulk quantities of raw milk (50). Kanamycin, gentamicin, amikacin, tigecycline, and ciprofloxacin can be suggested as the drugs of choice for foodborne diseases caused by Bacillus cereus, as other antibiotics used in this experiment have shown resistance against Bacillus cereus isolates.
CONCLUSIONS
Our research findings reveal that infant powdered milk and cereals available in local markets across Bangladesh are contaminated with Bacillus cereus. Furthermore, these isolates exhibit resistance to multiple antimicrobial agents. Consequently, antibiotic-resistant microorganisms in food or baby food products pose a significant public health risk, particularly for neonates. To mitigate the risk of foodborne illness in neonates consuming powdered infant formula (PIF), several recommendations have been proposed concerning preparation, storage, and management. Introducing Hazard Analysis and Critical Control Points (HACCP) at every stage of the food chain is recommended to control contamination from various pathogenic microorganisms. Additionally, implementing good manufacturing practices (GMP) on farms during production and milk storage is essential. These practices ensure that facilities are designed, constructed, and maintained to prevent the introduction of the Bacillus cereus group into raw milk and milk products.